Oxidation of guanine in G, GG, and GGG sequence contexts by aromatic pyrenyl radical cations and carbonate radical anions: relationship between kinetics and distribution of alkali-labile lesions
- PMID: 18211057
- PMCID: PMC3713794
- DOI: 10.1021/jp076777x
Oxidation of guanine in G, GG, and GGG sequence contexts by aromatic pyrenyl radical cations and carbonate radical anions: relationship between kinetics and distribution of alkali-labile lesions
Abstract
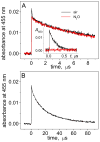
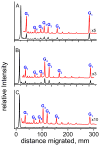
Oxidatively generated DNA damage induced by the aromatic radical cation of the pyrene derivative 7,8,9,10-tetrahydroxytetrahydrobenzo[a]pyrene (BPT), and by carbonate radicals anions, was monitored from the initial one-electron transfer, or hole injection step, to the formation of hot alkali-labile chemical end-products monitored by gel electrophoresis. The fractions of BPT molecules bound to double-stranded 20-35-mer oligonucleotides with noncontiguous guanines G and grouped as contiguous GG and GGG sequences were determined by a fluorescence quenching method. Utilizing intense nanosecond 355 nm Nd:YAG laser pulses, the DNA-bound BPT molecules were photoionized to BPT*+ radicals by a consecutive two-photon ionization mechanism. The BPT*+ radicals thus generated within the duplexes selectively oxidize guanine by intraduplex electron-transfer reactions, and the rate constants of these reactions follow the trend 5'-..GGG.. > 5'-..GG.. > 5'-..G... In the case of CO3*- radicals, the oxidation of guanine occurs by intermolecular collision pathways, and the bimolecular rate constants are independent of base sequence context. However, the distributions of the end-products generated by CO3*- radicals, as well as by BPT*+, are base sequence context-dependent and are greater than those in isolated guanines at the 5'-G in 5'-...GG... sequences, and the first two 5'- guanines in the 5'-..GGG sequences. These results help to clarify the conditions that lead to a similar or different base sequence dependence of the initial hole injection step and the final distribution of oxidized, alkali-labile guanine products. In the case of the intermolecular one-electron oxidant CO3*-, the rate constant of hole injection is similar for contiguous and isolated guanines, but the subsequent equilibration of holes by hopping favors trapping and product formation at contiguous guanines, and the sequence dependence of these two phenomena are not correlated. In contrast, in the case of the DNA-bound oxidant BPT*+, the hole injection rate constants, as well as hole equilibration, exhibit a similar dependence on base sequence context, and are thus correlated to one another.
Figures








Similar articles
-
Mechanisms of oxidation of guanine in DNA by carbonate radical anion, a decomposition product of nitrosoperoxycarbonate.Chemistry. 2007;13(16):4571-81. doi: 10.1002/chem.200601434. Chemistry. 2007. PMID: 17335089
-
Neighboring base sequence effect on DNA damage.J Biomol Struct Dyn. 2020 Jul;38(11):3188-3195. doi: 10.1080/07391102.2019.1659186. Epub 2019 Aug 28. J Biomol Struct Dyn. 2020. PMID: 31432766
-
DNA lesions derived from the site selective oxidation of Guanine by carbonate radical anions.Chem Res Toxicol. 2003 Dec;16(12):1528-38. doi: 10.1021/tx034142t. Chem Res Toxicol. 2003. PMID: 14680366
-
One-electron oxidation reactions of purine and pyrimidine bases in cellular DNA.Int J Radiat Biol. 2014 Jun;90(6):423-32. doi: 10.3109/09553002.2013.877176. Epub 2014 Apr 3. Int J Radiat Biol. 2014. PMID: 24369822 Free PMC article. Review.
-
The Two Faces of the Guanyl Radical: Molecular Context and Behavior.Molecules. 2021 Jun 9;26(12):3511. doi: 10.3390/molecules26123511. Molecules. 2021. PMID: 34207639 Free PMC article. Review.
Cited by
-
The fluorescence properties and lifetime study of G-quadruplexes single- and double-labeled with pyrene.J Fluoresc. 2010 Sep;20(5):1029-35. doi: 10.1007/s10895-010-0653-x. Epub 2010 Apr 1. J Fluoresc. 2010. PMID: 20358281
-
A systematic CRISPR screen defines mutational mechanisms underpinning signatures caused by replication errors and endogenous DNA damage.Nat Cancer. 2021 Jun;2(6):643-657. doi: 10.1038/s43018-021-00200-0. Epub 2021 Apr 26. Nat Cancer. 2021. PMID: 34164627 Free PMC article.
-
Proton-coupled hole hopping in nucleosomal and free DNA initiated by site-specific hole injection.Phys Chem Chem Phys. 2012 May 28;14(20):7400-10. doi: 10.1039/c2cp40759k. Epub 2012 Apr 24. Phys Chem Chem Phys. 2012. PMID: 22526555 Free PMC article.
-
PuF, an antimetastatic and developmental signaling protein, interacts with the Alzheimer's amyloid-β precursor protein via a tissue-specific proximal regulatory element (PRE).BMC Genomics. 2013 Jan 31;14:68. doi: 10.1186/1471-2164-14-68. BMC Genomics. 2013. PMID: 23368879 Free PMC article.
-
In situ analysis of 8-oxo-7,8-dihydro-2'-deoxyguanosine oxidation reveals sequence- and agent-specific damage spectra.J Am Chem Soc. 2012 Oct 31;134(43):18053-64. doi: 10.1021/ja307525h. Epub 2012 Oct 22. J Am Chem Soc. 2012. PMID: 23057664 Free PMC article.
References
-
- Beckman KB, Ames BN. J Biol Chem. 1997;272:19633–19636. - PubMed
-
- Dedon PC, Tannenbaum SR. Arch Biochem Biophys. 2004;423:12–22. - PubMed
-
- Nunez ME, Hall DB, Barton JK. Chem Biol. 1999;6:85–97. - PubMed
-
- Schuster GB. Acc Chem Res. 2000;33:253–260. - PubMed
-
- Giese B. Annu Rev Biochem. 2002;71:51–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

