Human CD34+ stem cells produce bone nodules in vivo
- PMID: 18211282
- PMCID: PMC6496867
- DOI: 10.1111/j.1365-2184.2007.00497.x
Human CD34+ stem cells produce bone nodules in vivo
Abstract
Objectives: The aim of this study was to select and provide enough stem cells for quick transplantation in bone engineering procedures, avoiding any in vitro expansion step.
Materials and methods: Dental germ pulp, collected from 25 healthy subjects aged 13-20 years, were subjected to magnetic-activated cell sorting to select a CD34(+) stem cell population capable of differentiating into pre-osteoblasts. These cells were allowed to adhere to an absorbable polylactic-coglycolic acid scaffold for 30 min, without any prior expansion, and the CD34(+) cell-colonized scaffolds were then transplanted into immunocompromised rats, subcutaneously.
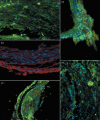
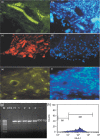
Results: After 60 days, analysis of recovered transplants revealed that they were formed of nodules of bone, of the same dimensions as the original scaffold. Bone-specific proteins were detected by immunofluorescence, within the nodules, and X-ray diffraction patterns revealed characteristic features of bone. In addition, presence of platelet endothelial cell adhesion molecule and von Willebrand factor immunoreactivity were suggestive of neo-angiogenesis and neovasculogenesis taking place within nodules. Importantly, these vessels were HLA-1(+) and, thus, clearly human in origin.
Conclusions: This study indicates that CD34(+) cells obtained from dental pulp can be used for engineering bone, without the need for prior culture expanding procedures. Using autologous stem cells, this schedule could be used to provide the basis for bone regenerative surgery, with limited sacrifice of tissue, low morbidity at the collection site, and significant reduction in time needed for clinical recovery.
Figures




Similar articles
-
A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB).J Bone Miner Res. 2005 Aug;20(8):1394-402. doi: 10.1359/JBMR.050325. Epub 2005 Mar 28. J Bone Miner Res. 2005. PMID: 16007337
-
Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation.Cell Death Differ. 2007 Jun;14(6):1162-71. doi: 10.1038/sj.cdd.4402121. Epub 2007 Mar 9. Cell Death Differ. 2007. PMID: 17347663
-
CD34 positive cells seeded on small caliber man-made vascular grafts exhibit increased antithrombogenic property compared with unfractioned mononuclear cells.J Cardiovasc Surg (Torino). 2010 Dec;51(6):885-94. J Cardiovasc Surg (Torino). 2010. PMID: 21124286
-
Cytokine-induced expansion of human CD34+ stem/progenitor and CD34+CD41+ early megakaryocytic marrow cells cultured on normal osteoblasts.Stem Cells. 1999;17(2):92-9. doi: 10.1002/stem.170092. Stem Cells. 1999. PMID: 10195569
-
Selection of stem cells by using antibodies that target different CD34 epitopes yields different patterns of T-cell differentiation.Stem Cells. 2007 Feb;25(2):537-42. doi: 10.1634/stemcells.2006-0319. Epub 2006 Oct 5. Stem Cells. 2007. PMID: 17023516
Cited by
-
The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review.J Tissue Eng. 2018 Jan 17;9:2041731417752766. doi: 10.1177/2041731417752766. eCollection 2018 Jan-Dec. J Tissue Eng. 2018. PMID: 29375756 Free PMC article. Review.
-
Reconstruction of Alar Nasal Cartilage Defects Using a Tissue Engineering Technique Based on a Combined Use of Autologous Chondrocyte Micrografts and Platelet-rich Plasma: Preliminary Clinical and Instrumental Evaluation.Plast Reconstr Surg Glob Open. 2016 Oct 26;4(10):e1027. doi: 10.1097/GOX.0000000000001027. eCollection 2016 Oct. Plast Reconstr Surg Glob Open. 2016. PMID: 27826462 Free PMC article.
-
Dental pulp stem cells as a multifaceted tool for bioengineering and the regeneration of craniomaxillofacial tissues.Front Physiol. 2015 Oct 16;6:289. doi: 10.3389/fphys.2015.00289. eCollection 2015. Front Physiol. 2015. PMID: 26528190 Free PMC article. Review.
-
Allogenic banking of dental pulp stem cells for innovative therapeutics.World J Stem Cells. 2015 Aug 26;7(7):1010-21. doi: 10.4252/wjsc.v7.i7.1010. World J Stem Cells. 2015. PMID: 26328017 Free PMC article. Review.
-
Dental pulp stem cells. Biology and use for periodontal tissue engineering.Saudi Med J. 2015 Dec;36(12):1391-9. doi: 10.15537/smj.2015.12.12750. Saudi Med J. 2015. PMID: 26620980 Free PMC article. Review.
References
-
- Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, Gazit D, Gazit Z (2006) Osteogenic differentiation of noncultured immunoisolated bone marrow‐derived CD105+ cells. Stem Cells 24, 1728–1737. - PubMed
-
- Barclay AN, Jackson DI, Willis AC, Williams AF (1988) The leukocyte‐common antigen (L‐CA) family. Adv. Exp. Med. Biol. 237, 3–7. - PubMed
-
- Boskey AL (1991) The role of extracellular matrix components in dentin mineralization. Crit. Rev. Oral Biol. Med. 2, 369–387. - PubMed
-
- Bosshardt DD (2005) Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J. Dent. Res. 84, 390–406. - PubMed
-
- Both SK, Muijsenberg AJ, Blitterswijk CA, Boer JD, Bruijn JD (2007) A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 13, 3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

