Biphasic onset of splenic apoptosis following hemorrhagic shock: critical implications for Bax, Bcl-2, and Mcl-1 proteins
- PMID: 18211685
- PMCID: PMC2374615
- DOI: 10.1186/cc6772
Biphasic onset of splenic apoptosis following hemorrhagic shock: critical implications for Bax, Bcl-2, and Mcl-1 proteins
Abstract
Introduction: The innate immune response to trauma hemorrhage involves inflammatory mediators, thus promoting cellular dysfunction as well as cell death in diverse tissues. These effects ultimately bear the risk of post-traumatic complications such as organ dysfunction, multiple organ failure, or adult respiratory distress syndrome. In this study, a murine model of resuscitated hemorrhagic shock (HS) was used to determine the apoptosis in spleen as a marker of cellular injury and reduced immune functions.
Methods: Male C57BL-6 mice were subjected to sham operation or resuscitated HS. At t = 0 hours, t = 24 hours, and t = 72 hours, mice were euthanized and the spleens were removed and evaluated for apoptotic changes via DNA fragmentation, caspase activities, and activation of both extrinsic and intrinsic apoptotic pathways. Spleens from untreated mice were used as control samples.
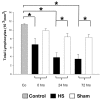
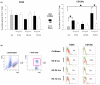
Results: HS was associated with distinct lymphocytopenia as early as t = 0 hours after hemorrhage without regaining baseline levels within the consecutive 72 hours when compared with sham and control groups. A rapid activation of splenic apoptosis in HS mice was observed at t = 0 hours and t = 72 hours after hemorrhage and predominantly confirmed by increased DNA fragmentation, elevated caspase-3/7, caspase-8, and caspase-9 activities, and enhanced expression of intrinsic mitochondrial proteins. Accordingly, mitochondrial pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins were inversely expressed within the 72-hour observation period, thereby supporting significant pro-apoptotic changes. Solely at t = 24 hours, expression of the anti-apoptotic Mcl-1 protein shows a significant increase when compared with sham-operated and control animals. Furthermore, expression of extrinsic death receptors were only slightly increased.
Conclusion: Our data suggest that HS induces apoptotic changes in spleen through a biphasic caspase-dependent mechanism and imply a detrimental imbalance of pro- and anti-apoptotic mitochondrial proteins Bax, Bcl-2, and Mcl-1, thereby promoting post-traumatic immunosuppression.
Figures


References
-
- Stephan RN, Kupper TS, Geha AS, Baue AE, Chaudry IH. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987;122:62–68. - PubMed
-
- Livingston DH, Malangoni MA. An experimental study of susceptibility to infection after hemorrhagic shock. Surg Gynecol Obstet. 1989;168:138–142. - PubMed
-
- Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. - PubMed
-
- Sasaki H, Matsuno T, Nakagawa K, Tanaka N. Induction of apoptosis during the early phase of reperfusion after rat liver ischemia. Acta Med Okayama. 1997;51:305–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

