Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system
- PMID: 18211716
- PMCID: PMC2249568
- DOI: 10.1186/1475-2859-7-1
Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system
Abstract
Background: Escherichia coli strains lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system (PTS) are capable of coutilizing glucose and other carbon sources due to the absence of catabolite repression by glucose. In these strains, the lack of this important regulatory and transport system allows the coexistence of glycolytic and gluconeogenic pathways. Strains lacking PTS have been constructed with the goal of canalizing part of the phosphoenolpyruvate (PEP) not consumed in glucose transport to the aromatic pathway. The deletion of the ptsHIcrr operon inactivates PTS causing poor growth on this sugar; nonetheless, fast growing mutants on glucose have been isolated (PB12 strain). However, there are no reported studies concerning the growth potential of a PTS- strain in mixtures of different carbon sources to enhance the production of aromatics compounds.
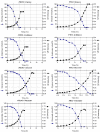
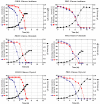
Results: PB12 strain is capable of coutilizing mixtures of glucose-arabinose, glucose-gluconate and glucose-glycerol. This capacity increases its specific growth rate (mu) given that this strain metabolizes more moles of carbon source per unit time. The presence of plasmids pRW300aroGfbr and pCLtktA reduces the mu of strain PB12 in all mixtures of carbon sources, but enhances the productivity and yield of aromatic compounds, especially in the glucose-glycerol mixture, as compared to glucose or glycerol cultures. No acetate was detected in the glycerol and the glucose-glycerol batch fermentations.
Conclusion: Due to the lack of catabolite repression, PB12 strain carrying multicopy plasmids containing tktA and aroGfbr genes is capable of coutilizing glucose and other carbon sources; this capacity, reduces its mu but increases the production of aromatic compounds.
Figures



Similar articles
-
Physiological and transcriptional characterization of Escherichia coli strains lacking interconversion of phosphoenolpyruvate and pyruvate when glucose and acetate are coutilized.Biotechnol Bioeng. 2014 Jun;111(6):1150-60. doi: 10.1002/bit.25177. Epub 2014 Jan 28. Biotechnol Bioeng. 2014. PMID: 24375081 Free PMC article.
-
Metabolic engineering for the production of shikimic acid in an evolved Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system.Microb Cell Fact. 2010 Apr 12;9:21. doi: 10.1186/1475-2859-9-21. Microb Cell Fact. 2010. PMID: 20385022 Free PMC article.
-
Adaptation for fast growth on glucose by differential expression of central carbon metabolism and gal regulon genes in an Escherichia coli strain lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system.Metab Eng. 2005 Mar;7(2):70-87. doi: 10.1016/j.ymben.2004.10.002. Metab Eng. 2005. PMID: 15781417
-
Inactivation of the PTS as a Strategy to Engineer the Production of Aromatic Metabolites in Escherichia coli.J Mol Microbiol Biotechnol. 2015;25(2-3):195-208. doi: 10.1159/000380854. Epub 2015 Jul 9. J Mol Microbiol Biotechnol. 2015. PMID: 26159079 Review.
-
Current knowledge of the Escherichia coli phosphoenolpyruvate-carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation.Appl Microbiol Biotechnol. 2012 Jun;94(6):1483-94. doi: 10.1007/s00253-012-4101-5. Epub 2012 May 11. Appl Microbiol Biotechnol. 2012. PMID: 22573269 Review.
Cited by
-
Combinatorial expression of different β-carotene hydroxylases and ketolases in Escherichia coli for increased astaxanthin production.J Ind Microbiol Biotechnol. 2019 Nov;46(11):1505-1516. doi: 10.1007/s10295-019-02214-1. Epub 2019 Jul 11. J Ind Microbiol Biotechnol. 2019. PMID: 31297712
-
Metabolic Engineering Strategies for Co-Utilization of Carbon Sources in Microbes.Bioengineering (Basel). 2016 Feb 6;3(1):10. doi: 10.3390/bioengineering3010010. Bioengineering (Basel). 2016. PMID: 28952572 Free PMC article. Review.
-
Metabolic modeling and response surface analysis of an Escherichia coli strain engineered for shikimic acid production.BMC Syst Biol. 2018 Nov 12;12(1):102. doi: 10.1186/s12918-018-0632-4. BMC Syst Biol. 2018. PMID: 30419897 Free PMC article.
-
Transcriptional and Metabolic Response of a Strain of Escherichia coli PTS- to a Perturbation of the Energetic Level by Modification of [ATP]/[ADP] Ratio.BioTech (Basel). 2024 Apr 10;13(2):10. doi: 10.3390/biotech13020010. BioTech (Basel). 2024. PMID: 38651490 Free PMC article.
-
Metabolic engineering for improving L-tryptophan production in Escherichia coli.J Ind Microbiol Biotechnol. 2019 Jan;46(1):55-65. doi: 10.1007/s10295-018-2106-5. Epub 2018 Nov 13. J Ind Microbiol Biotechnol. 2019. PMID: 30426284 Review.
References
-
- Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate: carbohydrate phosphotransferase systems. In: Neidhardt C, editor. Escherichia coli and Salmonella tiphymurium: Cellular and Molecular Biology. 2. Vol. 2. ASM Press, Washington, USA; 1996. pp. 1149–1174.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

