Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance
- PMID: 18212040
- PMCID: PMC2268427
- DOI: 10.1128/MCB.01490-07
Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance
Abstract
Dyskerin binds the H/ACA box of human telomerase RNA and is a core telomerase subunit required for RNP biogenesis and enzyme function in vivo. Missense mutations in dyskerin result in dyskeratosis congenita, a complex syndrome characterized by bone marrow failure, telomerase enzyme deficiency, and progressive telomere shortening. Here we demonstrate that dyskerin also contributes to telomere maintenance in Arabidopsis thaliana. We report that both AtNAP57, the Arabidopsis dyskerin homolog, and AtTERT, the telomerase catalytic subunit, accumulate in the plant nucleolus, and AtNAP57 associates with active telomerase RNP particles in an RNA-dependent manner. Furthermore, AtNAP57 interacts in vitro with AtPOT1a, a novel component of Arabidopsis telomerase. Although a null mutation in AtNAP57 is lethal, AtNAP57, like AtTERT, is not haploinsufficient for telomere maintenance in Arabidopsis. However, introduction of an AtNAP57 allele containing a T66A mutation decreased telomerase activity in vitro, disrupted telomere length regulation on individual chromosome ends in vivo, and established a new, shorter telomere length set point. These results imply that T66A NAP57 behaves as a dominant-negative inhibitor of telomerase. We conclude that dyskerin is a conserved component of the telomerase RNP complex in higher eukaryotes that is required for maximal enzyme activity in vivo.
Figures
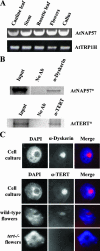
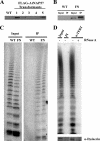


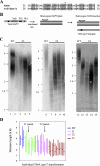
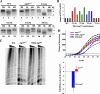
Similar articles
-
Arabidopsis retains vertebrate-type telomerase accessory proteins via a plant-specific assembly.Nucleic Acids Res. 2021 Sep 20;49(16):9496-9507. doi: 10.1093/nar/gkab699. Nucleic Acids Res. 2021. PMID: 34403479 Free PMC article.
-
The plant Pontin and Reptin homologues, RuvBL1 and RuvBL2a, colocalize with TERT and TRB proteins in vivo, and participate in telomerase biogenesis.Plant J. 2019 Apr;98(2):195-212. doi: 10.1111/tpj.14306. Epub 2019 Apr 9. Plant J. 2019. PMID: 30834599
-
Cloning and characterization of Arabidopsis thaliana AtNAP57--a homologue of yeast pseudouridine synthase Cbf5p.Acta Biochim Pol. 2001;48(3):699-709. Acta Biochim Pol. 2001. PMID: 11833778
-
Dyskerin: an essential pseudouridine synthase with multifaceted roles in ribosome biogenesis, splicing, and telomere maintenance.RNA. 2021 Dec;27(12):1441-1458. doi: 10.1261/rna.078953.121. Epub 2021 Sep 23. RNA. 2021. PMID: 34556550 Free PMC article. Review.
-
Dyskeratosis congenita: a disorder of defective telomere maintenance?Int J Hematol. 2005 Oct;82(3):184-9. doi: 10.1532/IJH97.05067. Int J Hematol. 2005. PMID: 16207588 Review.
Cited by
-
An alternative telomerase RNA in Arabidopsis modulates enzyme activity in response to DNA damage.Genes Dev. 2012 Nov 15;26(22):2512-23. doi: 10.1101/gad.202960.112. Epub 2012 Oct 29. Genes Dev. 2012. PMID: 23109676 Free PMC article.
-
Prevalent and distinct spliceosomal 3'-end processing mechanisms for fungal telomerase RNA.Nat Commun. 2015 Jan 19;6:6105. doi: 10.1038/ncomms7105. Nat Commun. 2015. PMID: 25598218 Free PMC article.
-
Plant telomere biology: The green solution to the end-replication problem.Plant Cell. 2022 Jul 4;34(7):2492-2504. doi: 10.1093/plcell/koac122. Plant Cell. 2022. PMID: 35511166 Free PMC article.
-
Telomere repeat binding proteins are functional components of Arabidopsis telomeres and interact with telomerase.Plant J. 2014 Mar;77(5):770-81. doi: 10.1111/tpj.12428. Epub 2014 Feb 18. Plant J. 2014. PMID: 24397874 Free PMC article.
-
Single-cell telomere-length quantification couples telomere length to meristem activity and stem cell development in Arabidopsis.Cell Rep. 2015 May 12;11(6):977-989. doi: 10.1016/j.celrep.2015.04.013. Epub 2015 Apr 30. Cell Rep. 2015. PMID: 25937286 Free PMC article.
References
-
- Armanios, M., J. L. Chen, Y. P. Chang, R. A. Brodsky, A. Hawkins, C. A. Griffin, J. R. Eshleman, A. R. Cohen, A. Chakravarti, A. Hamosh, and C. W. Greider. 2005. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl. Acad. Sci. USA 10215960-15964. - PMC - PubMed
-
- Armstrong, S. J., F. C. Franklin, and G. H. Jones. 2001. Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. J. Cell Sci. 1144207-4217. - PubMed
-
- Baumann, P. 2006. Are mouse telomeres going to pot? Cell 12633-36. - PubMed
-
- Cerone, M. A., R. J. Ward, J. A. Londono-Vallejo, and C. Autexier. 2005. Telomerase RNA mutated in autosomal dyskeratosis congenita reconstitutes a weakly active telomerase enzyme defective in telomere elongation. Cell Cycle 4585-589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
