Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance
- PMID: 18212043
- PMCID: PMC2268419
- DOI: 10.1128/MCB.00998-07
Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance
Abstract
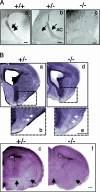
The chemotropic guidance cue netrin-1 promotes neurite outgrowth through its receptor Deleted in Colorectal Cancer (DCC) via activation of Rac1. The guanine nucleotide exchange factor (GEF) linking netrin-1/DCC to Rac1 activation has not yet been identified. Here, we show that the RhoGEF Trio mediates Rac1 activation in netrin-1 signaling. We found that Trio interacts with the netrin-1 receptor DCC in mouse embryonic brains and that netrin-1-induced Rac1 activation in brain is impaired in the absence of Trio. Trio(-/-) cortical neurons fail to extend neurites in response to netrin-1, while they are able to respond to glutamate. Accordingly, netrin-1-induced commissural axon outgrowth is reduced in Trio(-/-) spinal cord explants, and the guidance of commissural axons toward the floor plate is affected by the absence of Trio. The anterior commissure is absent in Trio-null embryos, and netrin-1/DCC-dependent axonal projections that form the internal capsule and the corpus callosum are defective in the mutants. Taken together, these findings establish Trio as a GEF that mediates netrin-1 signaling in axon outgrowth and guidance through its ability to activate Rac1.
Figures
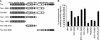

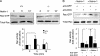



References
-
- Ackerman, S. L., L. P. Kozak, S. A. Przyborski, L. A. Rund, B. B. Boyer, and B. B. Knowles. 1997. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386838-842. - PubMed
-
- Awasaki, T., M. Saito, M. Sone, E. Suzuki, R. Sakai, K. Ito, and C. Hama. 2000. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 26119-131. - PubMed
-
- Barallobre, M. J., M. Pascual, J. A. Del Rio, and E. Soriano. 2005. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res. Brain Res. Rev. 4922-47. - PubMed
-
- Bateman, J., H. Shu, and D. Van Vactor. 2000. The guanine nucleotide exchange factor Trio mediates axonal development in the Drosophila embryo. Neuron 2693-106. - PubMed
-
- Bellanger, J. M., J. B. Lazaro, S. Diriong, A. Fernandez, N. Lamb, and A. Debant. 1998. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene 16147-152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
