Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism
- PMID: 18212053
- PMCID: PMC2268413
- DOI: 10.1128/MCB.01780-07
Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism
Abstract
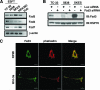
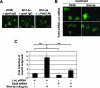
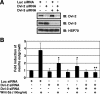
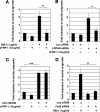
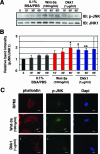
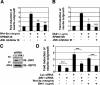
Recombinant Wnt-3a stimulated the rapid formation of elongated processes in Ewing sarcoma family tumor (ESFT) cells that were identified as neurites. The processes stained positively for polymerized actin and microtubules as well as synapsin I and growth-associated protein 43. Inhibition of the Wnt receptor, Frizzled3 (Fzd3), with antiserum or by short interfering RNA (siRNA) markedly reduced neurite extension. Knockdown of Dishevelled-2 (Dvl-2) and Dvl-3 also suppressed neurite outgrowth. Surprisingly, disruption of the Wnt/Fzd/lipoprotein receptor-related protein (LRP) complex and the associated beta-catenin signaling by treating cells either with the Wnt antagonist Dickkopf-1 (Dkk1) or LRP5/LRP6 siRNA enhanced neuritogenesis. Neurite outgrowth induced by Dkk1 or with LRP5/LRP6 siRNA was inhibited by secreted Fzd-related protein 1, a Wnt antagonist that binds directly to Wnt. Moreover, Dkk1 stimulation of neurite outgrowth was blocked by Fzd3 siRNA. These results suggested that Dkk1 shifted endogenous Wnt activity from the beta-catenin pathway to Fzd3-mediated, noncanonical signaling that is responsible for neurite formation. In particular, c-Jun amino-terminal kinase (JNK) was important for neurite outgrowth stimulated by both Wnt-3a and Dkk1. Our data demonstrate that Fzd3, Dvl, and JNK activity mediate Wnt-dependent neurite outgrowth and that ESFT cell lines will be useful experimental models for the study of Wnt-dependent neurite extension.
Figures


References
-
- Bovolenta, P., J. Rodriguez, and P. Esteve. 2006. Frizzled/RYK mediated signalling in axon guidance. Development 1334399-4408. - PubMed
-
- Caneparo, L., Y. L. Huang, N. Staudt, M. Tada, R. Ahrendt, O. Kazanskaya, C. Niehrs, and C. Houart. 2007. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev. 21465-480. - PMC - PubMed
-
- Chang, L., Y. Jones, M. H. Ellisman, L. S. Goldstein, and M. Karin. 2003. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4521-533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
