Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs
- PMID: 18212087
- PMCID: PMC2258824
- DOI: 10.1128/IAI.01011-07
Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs
Abstract
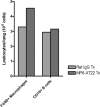
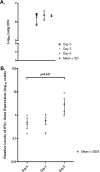
Tumor necrosis factor (TNF) is a prototypic proinflammatory cytokine that contributes significantly to the development of immunopathology in various disease states. A complication of TNF blockade therapy, which is used increasingly for the treatment of chronic inflammatory diseases, is the reactivation of latent tuberculosis. This study used a low-dose aerogenic model of murine tuberculosis to analyze the effect of TNF neutralization on disease progression in mice with chronic tuberculous infections. Histological, immunohistochemical, and flow cytometric analyses of Mycobacterium tuberculosis-infected lung tissues revealed that the neutralization of TNF results in marked disorganization of the tuberculous granuloma, as demonstrated by the dissolution of the previously described B-cell-macrophage unit in granulomatous tissues as well as by increased inflammatory cell infiltration. Quantitative gene expression studies using laser capture microdissected granulomatous lung tissues revealed that TNF blockade in mice chronically infected with M. tuberculosis leads to the enhanced expression of specific proinflammatory molecules. Collectively, these studies have provided evidence suggesting that in the chronic phase of M. tuberculosis infection, TNF is essential for maintaining the structure of the tuberculous granuloma and may regulate the granulomatous response by exerting an anti-inflammatory effect through modulation of the expression of proinflammatory mediators.
Figures





References
-
- Algood, H. M., J. Chan, and J. L. Flynn. 2003. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 14467-477. - PubMed
-
- Algood, H. M., P. L. Lin, and J. L. Flynn. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 41(Suppl. 3)S189-S193. - PubMed
-
- Algood, H. M., P. L. Lin, D. Yankura, A. Jones, J. Chan, and J. L. Flynn. 2004. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J. Immunol. 1726846-6857. - PubMed
-
- Aloisi, F., and R. Pujol-Borrell. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6205-217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

