Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice
- PMID: 18212290
- PMCID: PMC4007137
- DOI: 10.1161/CIRCULATIONAHA.107.717595
Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice
Abstract
Background: Fat inflammation may play an important role in comorbidities associated with obesity such as atherosclerosis.
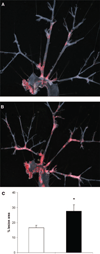
Methods and results: To first establish feasibility of fat transplantation, epididymal fat pads were harvested from wild-type C57BL/6J mice and transplanted into leptin-deficient (Lep(ob/ob)) mice. Fat transplantation produced physiological leptin levels and prevented obesity and infertility in Lep(ob/ob) mice. However, the transplanted fat depots were associated with chronically increased macrophage infiltration with characteristics identical to those observed in fat harvested from obese animals. The inflammation in transplanted adipose depots was regulated by the same factors that have been implicated in endogenous fat inflammation such as monocyte chemoattractant protein-1. To determine whether this inflamed adipose depot could affect vascular disease in mice, epididymal fat depots were transplanted into atherosclerosis-prone apolipoprotein E-deficient ApoE(-/-) mice. Plasma from ApoE(-/-) mice receiving fat transplants contained increased leptin, resistin, and monocyte chemoattractant protein-1 compared with plasma from sham-operated ApoE(-/-) mice. Furthermore, mice transplanted with visceral fat developed significantly more atherosclerosis compared with sham-operated animals, whereas transplants with subcutaneous fat did not affect atherosclerosis despite a similar degree of fat inflammation. Treatment of transplanted ApoE(-/-) mice with pioglitazone decreased macrophage content of the transplanted visceral fat pad and reduced plasma monocyte chemoattractant protein-1. Importantly, pioglitazone also reduced atherosclerosis triggered by inflammatory visceral fat but had no protective effect on atherosclerosis in the absence of the visceral fat transplantation.
Conclusions: Our results indicate that visceral adipose-related inflammation accelerates atherosclerosis in mice. Drugs such as thiazolidinediones might be a useful strategy to specifically attenuate the vascular disease induced by visceral inflammatory fat.
Figures



References
-
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. - PubMed
-
- Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:136–147. - PubMed
-
- See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–759. - PubMed
-
- Lakka HM, Lakka TA, Tuomilehto J, Salonen JT. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur Heart J. 2002;23:706–713. - PubMed
-
- Blaschke F, Spanheimer R, Khan M, Law RE. Vascular effects of TZDs: new implications. Vasc Pharmacol. 2006;45:3–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

