Conditional immortalization of human B cells by CD40 ligation
- PMID: 18213373
- PMCID: PMC2180193
- DOI: 10.1371/journal.pone.0001464
Conditional immortalization of human B cells by CD40 ligation
Abstract
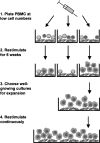
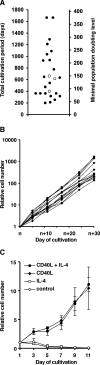
It is generally assumed that human differentiated cells have a limited life-span and proliferation capacity in vivo, and that genetic modifications are a prerequisite for their immortalization in vitro. Here we readdress this issue, studying the long-term proliferation potential of human B cells. It was shown earlier that human B cells from peripheral blood of healthy donors can be efficiently induced to proliferate for up to ten weeks in vitro by stimulating their receptor CD40 in the presence of interleukin-4. When we applied the same stimuli under conditions of modified cell number and culture size, we were surprised to find that our treatment induced B cells to proliferate throughout an observation period of presently up to 1650 days, representing more than 370 population doublings, which suggested that these B cells were immortalized in vitro. Long-term CD40-stimulated B cell cultures could be established from most healthy adult human donors. These B cells had a constant phenotype, were free from Epstein-Barr virus, and remained dependent on CD40 ligation. They had constitutive telomerase activity and stabilized telomere length. Moreover, they were susceptible to activation by Toll-like receptor 9 ligands, and could be used to expand antigen-specific cytotoxic T cells in vitro. Our results indicate that human somatic cells can evade senescence and be conditionally immortalized by external stimulation only, without a requirement for genetic manipulation or oncoviral infection. Conditionally immortalized human B cells are a new tool for immunotherapy and studies of B cell oncogenesis, activation, and function.
Conflict of interest statement
Figures







Comment in
-
Immortalized B cells: a neverending source of antigen-presenting cells for vaccines?Expert Rev Vaccines. 2008 May;7(4):411-5. doi: 10.1586/14760584.7.4.411. Expert Rev Vaccines. 2008. PMID: 18444888 Review. No abstract available.
References
-
- Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. - PubMed
-
- Stewart SA, Weinberg RA. Telomeres: cancer to human aging. Annu Rev Cell Dev Biol. 2006;22:531–557. - PubMed
-
- Norrback KF, Dahlenborg K, Carlsson R, Roos G. Telomerase activation in normal B lymphocytes and non-Hodgkin's lymphomas. Blood. 1996;88:222–229. - PubMed
-
- Igarashi H, Sakaguchi N. Telomerase activity is induced in human peripheral B lymphocytes by the stimulation to antigen receptor. Blood. 1997;89:1299–1307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

