Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial
- PMID: 18213374
- PMCID: PMC2186380
- DOI: 10.1371/journal.pone.0001465
Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial
Abstract
Background: The objective was to evaluate the safety, reactogenicity and immunogenicity of the AMA-1-based blood-stage malaria vaccine FMP2.1/AS02A in adults exposed to seasonal malaria.
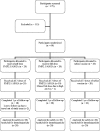
Methodology/principal findings: A phase 1 double blind randomized controlled dose escalation trial was conducted in Bandiagara, Mali, West Africa, a rural town with intense seasonal transmission of Plasmodium falciparum malaria. The malaria vaccine FMP2.1/AS02A is a recombinant protein (FMP2.1) based on apical membrane antigen-1 (AMA-1) from the 3D7 clone of P. falciparum, adjuvanted with AS02A. The comparator vaccine was a cell-culture rabies virus vaccine (RabAvert). Sixty healthy, malaria-experienced adults aged 18-55 y were recruited into 2 cohorts and randomized to receive either a half dose or full dose of the malaria vaccine (FMP2.1 25 microg/AS02A 0.25 mL or FMP2.1 50 microg/AS02A 0.5 mL) or rabies vaccine given in 3 doses at 0, 1 and 2 mo, and were followed for 1 y. Solicited symptoms were assessed for 7 d and unsolicited symptoms for 30 d after each vaccination. Serious adverse events were assessed throughout the study. Titers of anti-AMA-1 antibodies were measured by ELISA and P. falciparum growth inhibition assays were performed on sera collected at pre- and post-vaccination time points. Transient local pain and swelling were common and more frequent in both malaria vaccine dosage groups than in the comparator group. Anti-AMA-1 antibodies increased significantly in both malaria vaccine groups, peaking at nearly 5-fold and more than 6-fold higher than baseline in the half-dose and full-dose groups, respectively.
Conclusion/significance: The FMP2.1/AS02A vaccine had a good safety profile, was well-tolerated, and was highly immunogenic in malaria-exposed adults. This malaria vaccine is being evaluated in Phase 1 and 2 trials in children at this site.
Trial registration: ClinicalTrials.gov NCT00308061.
Conflict of interest statement
Figures


References
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. - PubMed
-
- Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. - PubMed
-
- Heppner DG, Jr, Kester KE, Ockenhouse CF, Tornieporth N, Ofori O, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23:2243–2250. - PubMed
-
- Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun. 2007;75:2283–2290. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

