A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children
- PMID: 18213379
- PMCID: PMC2198946
- DOI: 10.1371/journal.pone.0001471
A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children
Abstract
In the Sahel, most malaria deaths occur among children 1-4 years old during a short transmission season. A trial of seasonal intermittent preventive treatment (IPT) with sulfadoxine-pyrimethamine (SP) and a single dose of artesunate (AS) showed an 86% reduction in the incidence of malaria in Senegal but this may not be the optimum regimen. We compared this regimen with three alternatives.
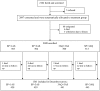
Methods: 2102 children aged 6-59 months received either one dose of SP plus one dose of AS (SP+1AS) (the previous regimen), one dose of SP plus 3 daily doses of AS (SP+3AS), one dose of SP plus three daily doses of amodiaquine (AQ) (SP+3AQ) or 3 daily doses of AQ and AS (3AQ+3AS). Treatments were given once a month on three occasions during the malaria transmission season. The primary end point was incidence of clinical malaria. Secondary end-points were incidence of adverse events, mean haemoglobin concentration and prevalence of parasites carrying markers of resistance to SP.
Findings: The incidence of malaria, and the prevalence of parasitaemia at the end of the transmission season, were lowest in the group that received SP+3AQ: 10% of children in the group that received SP+1AS had malaria, compared to 9% in the SP+3AS group (hazard ratio HR 0.90, 95%CI 0.60, 1.36); 11% in the 3AQ+3AS group, HR 1.1 (0.76-1.7); and 5% in the SP+3AQ group, HR 0.50 (0.30-0.81). Mutations associated with resistance to SP were present in almost all parasites detected at the end of the transmission season, but the prevalence of Plasmodium falciparum was very low in the SP+3AQ group.
Conclusions: Monthly treatment with SP+3AQ is a highly effective regimen for seasonal IPT. Choice of this regimen would minimise the spread of drug resistance and allow artemisinins to be reserved for the treatment of acute clinical malaria.
Trial registration: ClinicalTrials.gov NCT00132548.
Conflict of interest statement
Figures
References
-
- Bremen J. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 2001;(64 supplement):1–11. - PubMed
-
- Lengeler C. The Cochrane Library, Issue 4. Oxford: Update Software; 2001. Insecticide-treated bednets and curtains for preventing malaria (Cochrane Review). - PubMed
-
- Greenwood BM. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70:1–7. - PubMed
-
- Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo- controlled trial. Lancet. 2001;357:1471–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials