Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling
- PMID: 18213395
- PMCID: PMC2198940
- DOI: 10.1371/journal.pone.0001487
Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling
Abstract
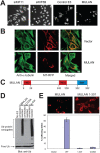
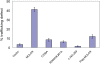
Specificity of protein ubiquitylation is conferred by E3 ubiquitin (Ub) ligases. We have annotated approximately 617 putative E3s and substrate-recognition subunits of E3 complexes encoded in the human genome. The limited knowledge of the function of members of the large E3 superfamily prompted us to generate genome-wide E3 cDNA and RNAi expression libraries designed for functional screening. An imaging-based screen using these libraries to identify E3s that regulate mitochondrial dynamics uncovered MULAN/FLJ12875, a RING finger protein whose ectopic expression and knockdown both interfered with mitochondrial trafficking and morphology. We found that MULAN is a mitochondrial protein - two transmembrane domains mediate its localization to the organelle's outer membrane. MULAN is oriented such that its E3-active, C-terminal RING finger is exposed to the cytosol, where it has access to other components of the Ub system. Both an intact RING finger and the correct subcellular localization were required for regulation of mitochondrial dynamics, suggesting that MULAN's downstream effectors are proteins that are either integral to, or associated with, mitochondria and that become modified with Ub. Interestingly, MULAN had previously been identified as an activator of NF-kappaB, thus providing a link between mitochondrial dynamics and mitochondria-to-nucleus signaling. These findings suggest the existence of a new, Ub-mediated mechanism responsible for integration of mitochondria into the cellular environment.
Conflict of interest statement
Figures



References
-
- Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. - PubMed
-
- Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. - PubMed
-
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. - PubMed
-
- Koppen M, Langer T. Protein Degradation within Mitochondria: Versatile Activities of AAA Proteases and Other Peptidases. Crit Rev Biochem Mol Biol. 2007;42:221–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

