Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice
- PMID: 18215233
- PMCID: PMC2376819
- DOI: 10.1111/j.1460-9568.2008.06024.x
Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice
Abstract
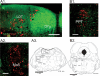
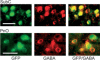
Recent experiments suggest that brainstem GABAergic neurons may control rapid-eye-movement (REM) sleep. However, understanding their pharmacology/physiology has been hindered by difficulty in identification. Here we report that mice expressing green fluorescent protein (GFP) under the control of the GAD67 promoter (GAD67-GFP knock-in mice) exhibit numerous GFP-positive neurons in the central gray and reticular formation, allowing on-line identification in vitro. Small (10-15 microm) or medium-sized (15-25 microm) GFP-positive perikarya surrounded larger serotonergic, noradrenergic, cholinergic and reticular neurons, and > 96% of neurons were double-labeled for GFP and GABA, confirming that GFP-positive neurons are GABAergic. Whole-cell recordings in brainstem regions important for promoting REM sleep [subcoeruleus (SubC) or pontine nucleus oralis (PnO) regions] revealed that GFP-positive neurons were spontaneously active at 3-12 Hz, fired tonically, and possessed a medium-sized depolarizing sag during hyperpolarizing steps. Many neurons also exhibited a small, low-threshold calcium spike. GFP-positive neurons were tested with pharmacological agents known to promote (carbachol) or inhibit (orexin A) REM sleep. SubC GFP-positive neurons were excited by the cholinergic agonist carbachol, whereas those in the PnO were either inhibited or excited. GFP-positive neurons in both areas were excited by orexins/hypocretins. These data are congruent with the hypothesis that carbachol-inhibited GABAergic PnO neurons project to, and inhibit, REM-on SubC reticular neurons during waking, whereas carbachol-excited SubC and PnO GABAergic neurons are involved in silencing locus coeruleus and dorsal raphe aminergic neurons during REM sleep. Orexinergic suppression of REM during waking is probably mediated in part via excitation of acetylcholine-inhibited GABAergic neurons.
Figures






References
-
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J. Neurosci. 2005;25:7406–7419. - PMC - PubMed
-
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 1996;229:891–895. - PubMed
-
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur. J. Neurosci. 2002;16:1959–1973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

