Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships
- PMID: 18215274
- PMCID: PMC2259306
- DOI: 10.1186/1471-2148-8-16
Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships
Abstract
Background: The neprilysin (M13) family of endopeptidases are zinc-metalloenzymes, the majority of which are type II integral membrane proteins. The best characterised of this family is neprilysin, which has important roles in inactivating signalling peptides involved in modulating neuronal activity, blood pressure and the immune system. Other family members include the endothelin converting enzymes (ECE-1 and ECE-2), which are responsible for the final step in the synthesis of potent vasoconstrictor endothelins. The ECEs, as well as neprilysin, are considered valuable therapeutic targets for treating cardiovascular disease. Other members of the M13 family have not been functionally characterised, but are also likely to have biological roles regulating peptide signalling. The recent sequencing of animal genomes has greatly increased the number of M13 family members in protein databases, information which can be used to reveal evolutionary relationships and to gain insight into conserved biological roles.
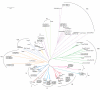
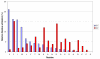
Results: The phylogenetic analysis successfully resolved vertebrate M13 peptidases into seven classes, one of which appears to be specific to mammals, and insect genes into five functional classes and a series of expansions, which may include inactive peptidases. Nematode genes primarily resolved into groups containing no other taxa, bar the two nematode genes associated with Drosophila DmeNEP1 and DmeNEP4. This analysis reconstructed only one relationship between chordate and invertebrate clusters, that of the ECE sub-group and the DmeNEP3 related genes. Analysis of amino acid utilisation in the active site of M13 peptidases reveals a basis for their biochemical properties. A relatively invariant S1' subsite gives the majority of M13 peptidases their strong preference for hydrophobic residues in P1' position. The greater variation in the S2' subsite may be instrumental in determining the specificity of M13 peptidases for their substrates and thus allows M13 peptidases to fulfil a broad range of physiological roles.
Conclusion: The M13 family of peptidases have diversified extensively in all species examined, indicating wide ranging roles in numerous physiological processes. It is predicted that differences in the S2' subsite are fundamental to determining the substrate specificities that facilitate this functional diversity.
Figures

References
-
- Turner AJ. In: Handbook of Proteolytic Enzymes. 2nd. Barret AJ, Rawlings ND, Woessner JF, editor. Vol. 1. London , Elsevier academic press; 2004. Neprilysin; pp. 419–425.
-
- Carpentier M, Desgroseillers L, Boileau G. In: Handbook of Proteolytic Enzymes. 2nd. Barret A, Rawlings ND, Woessner JF, editor. Vol. 1. London , Esevier academic press; 2004. Neprilysin-2; pp. 426–429.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

