Lactation failure in Src knockout mice is due to impaired secretory activation
- PMID: 18215306
- PMCID: PMC2266720
- DOI: 10.1186/1471-213X-8-6
Lactation failure in Src knockout mice is due to impaired secretory activation
Abstract
Background: Mammary gland development culminates in lactation and is orchestrated by numerous stimuli and signaling pathways. The Src family of nonreceptor tyrosine kinases plays a pivotal role in cell signaling. In order to determine if Src plays a role in mammary gland development we have examined mammary gland development and function during pregnancy and lactation in mice in which expression of Src has been eliminated.
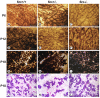
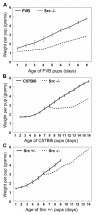
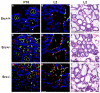
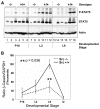
Results: We have characterized a lactation defect in the Src-/- mice which results in the death of over 80% of the litters nursed by Src-/- dams. Mammary gland development during pregnancy appears normal in these mice; however secretory activation does not seem to occur. Serum prolactin levels are normal in Src-/- mice compared to wildtype controls. Expression of the prolactin receptor at both the RNA and protein level was decreased in Src-/- mice following the transition from pregnancy to lactation, as was phosphorylation of STAT5 and expression of milk protein genes. These results suggest that secretory activation, which occurs following parturition, does not occur completely in Src-/- mice. Failed secretory activation results in precocious involution in the mammary glands of Src-/- even when pups were suckling. Involution was accelerated following pup withdrawal perhaps as a result of incomplete secretory activation. In vitro differentiation of mammary epithelial cells from Src-/- mice resulted in diminished production of milk proteins compared to the amount of milk proteins produced by Src+/+ cells, indicating a direct role for Src in regulating the transcription/translation of milk protein genes in mammary epithelial cells.
Conclusion: Src is an essential signaling modulator in mammary gland development as Src-/- mice exhibit a block in secretory activation that results in lactation failure and precocious involution. Src appears to be required for increased expression of the prolactin receptor and successful downstream signaling, and alveolar cell organization.
Figures






Similar articles
-
CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy.PLoS Genet. 2017 Mar 9;13(3):e1006654. doi: 10.1371/journal.pgen.1006654. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28278176 Free PMC article.
-
Zinc Finger Homeodomain Factor Zfhx3 Is Essential for Mammary Lactogenic Differentiation by Maintaining Prolactin Signaling Activity.J Biol Chem. 2016 Jun 10;291(24):12809-12820. doi: 10.1074/jbc.M116.719377. Epub 2016 Apr 20. J Biol Chem. 2016. PMID: 27129249 Free PMC article.
-
Transcriptional changes underlying the secretory activation phase of mammary gland development.Mol Endocrinol. 2005 Jul;19(7):1868-83. doi: 10.1210/me.2004-0254. Epub 2005 Feb 10. Mol Endocrinol. 2005. PMID: 15705664
-
STAT5-Driven Enhancers Tightly Control Temporal Expression of Mammary-Specific Genes.J Mammary Gland Biol Neoplasia. 2019 Mar;24(1):61-71. doi: 10.1007/s10911-018-9418-y. Epub 2018 Oct 17. J Mammary Gland Biol Neoplasia. 2019. PMID: 30328555 Review.
-
The role of prolactin and growth hormone in mammary gland development.Mol Cell Endocrinol. 2002 Nov 29;197(1-2):127-31. doi: 10.1016/s0303-7207(02)00286-1. Mol Cell Endocrinol. 2002. PMID: 12431805 Review.
Cited by
-
Signal transducer and activator of transcription 5 as a key signaling pathway in normal mammary gland developmental biology and breast cancer.Breast Cancer Res. 2011 Oct 12;13(5):220. doi: 10.1186/bcr2921. Breast Cancer Res. 2011. PMID: 22018398 Free PMC article. Review.
-
Mouse mammary tumor virus suppresses apoptosis of mammary epithelial cells through ITAM-mediated signaling.J Virol. 2012 Dec;86(24):13232-40. doi: 10.1128/JVI.02029-12. Epub 2012 Sep 26. J Virol. 2012. PMID: 23015704 Free PMC article.
-
Inhibition of the Activity of Cyclophilin A Impedes Prolactin Receptor-Mediated Signaling, Mammary Tumorigenesis, and Metastases.iScience. 2020 Sep 19;23(10):101581. doi: 10.1016/j.isci.2020.101581. eCollection 2020 Oct 23. iScience. 2020. PMID: 33083747 Free PMC article.
-
Genetic Diversity, Population Structure and Selection Signature in Begait Goats Revealed by Whole-Genome Sequencing.Animals (Basel). 2024 Jan 18;14(2):307. doi: 10.3390/ani14020307. Animals (Basel). 2024. PMID: 38254476 Free PMC article.
-
Rapid Communication: Genome-wide association analyses identify loci associated with colostrum production in Jersey cattle1.J Anim Sci. 2019 Mar 1;97(3):1117-1123. doi: 10.1093/jas/sky482. J Anim Sci. 2019. PMID: 30576450 Free PMC article.
References
-
- Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–186. - PubMed
-
- Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ. Prolactin Regulation of Mammary Gland Development. J Mammary Gland Biol Neoplasia. 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

