hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions
- PMID: 18215318
- PMCID: PMC2266713
- DOI: 10.1186/1471-2148-8-19
hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions
Abstract
Background: Hsp70 chaperones are required for key cellular processes and response to environmental changes and survival but they have not been fully characterized yet. The human hsp70-gene family has an unknown number of members (eleven counted over ten years ago); some have been described but the information is incomplete and inconsistent. A coherent body of knowledge encompassing all family components that would facilitate their study individually and as a group is lacking. Nowadays, the study of chaperone genes benefits from the availability of genome sequences and a new protocol, chaperonomics, which we applied to elucidate the human hsp70 family.
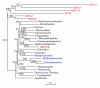
Results: We identified 47 hsp70 sequences, 17 genes and 30 pseudogenes. The genes distributed into seven evolutionarily distinct groups with distinguishable subgroups according to phylogenetic and other data, such as exon-intron and protein features. The N-terminal ATP-binding domain (ABD) was conserved at least partially in the majority of the proteins but the C-terminal substrate-binding domain (SBD) was not. Nine proteins were typical Hsp70s (65-80 kDa) with ABD and SBD, two were lighter lacking partly or totally the SBD, and six were heavier (>80 kDa) with divergent C-terminal domains. We also analyzed exon-intron features, transcriptional variants and protein structure and isoforms, and modality and patterns of expression in various tissues and developmental stages. Evolutionary analyses, including human hsp70 genes and pseudogenes, and other eukaryotic hsp70 genes, showed that six human genes encoding cytosolic Hsp70s and 27 pseudogenes originated from retro-transposition of HSPA8, a gene highly expressed in most tissues and developmental stages.
Conclusion: The human hsp70-gene family is characterized by a remarkable evolutionary diversity that mainly resulted from multiple duplications and retrotranspositions of a highly expressed gene, HSPA8. Human Hsp70 proteins are clustered into seven evolutionary Groups, with divergent C-terminal domains likely defining their distinctive functions. These functions may also be further defined by the observed differences in the N-terminal domain.
Figures





References
-
- Mayer MP, Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol Chem. 1998;379:261–268. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

