Human embryonic stem cell (hES) derived dendritic cells are functionally normal and are susceptible to HIV-1 infection
- PMID: 18215326
- PMCID: PMC2248203
- DOI: 10.1186/1742-6405-5-1
Human embryonic stem cell (hES) derived dendritic cells are functionally normal and are susceptible to HIV-1 infection
Abstract
Background: Human embryonic stem (hES) cells hold considerable promise for cell replacement and gene therapies. Their remarkable properties of pluripotency, self-renewal, and tractability for genetic modification potentially allows for the production of sizeable quantities of therapeutic cells of the hematopoietic lineage. Dendritic cells (DC) arise from CD34+ hematopoietic progenitor cells (HPCs) and are important in many innate and adaptive immune functions. With respect to HIV-1 infection, DCs play an important role in the efficient capture and transfer of the virus to susceptible cells. With an aim of generating DCs from a renewable source for HIV-1 studies, here we evaluated the capacity of hES cell derived CD34+ cells to give rise to DCs which can support HIV-1 infection.
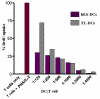
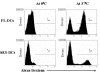
Results: Undifferentiated hES cells were cultured on S17 mouse bone marrow stromal cell layers to derive CD34+ HPCs which were subsequently grown in specific cytokine differentiation media to promote the development of DCs. The hES derived DCs (hES-DC) were subjected to phenotypic and functional analyses and compared with DCs derived from fetal liver CD34+ HPC (FL-DC). The mature hES-DCs displayed typical DC morphology consisting of veiled stellate cells. The hES-DCs also displayed characteristic phenotypic surface markers CD1a, HLA-DR, B7.1, B7.2, and DC-SIGN. The hES-DCs were found to be capable of antigen uptake and stimulating naïve allogeneic CD4+ T cells in a mixed leukocyte reaction assay. Furthermore, the hES-DCs supported productive HIV-1 viral infection akin to standard DCs.
Conclusion: Phenotypically normal and functionally competent DCs that support HIV-1 infection can be derived from hES cells. hES-DCs can now be exploited in applied immunology and HIV-1 infection studies. Using gene therapy approaches, it is now possible to generate HIV-1 resistant DCs from anti-HIV gene transduced hES-CD34+ hematopoietic progenitor cells.
Figures


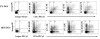

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

