Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD
- PMID: 18215622
- PMCID: PMC3147180
- DOI: 10.1016/j.neuron.2007.11.027
Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD
Abstract
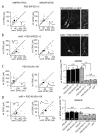
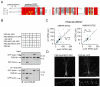
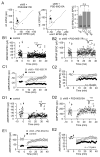
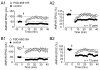
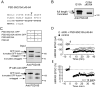
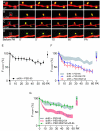
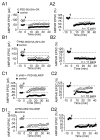
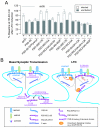
The postsynaptic density protein PSD-95 influences synaptic AMPA receptor (AMPAR) content and may play a critical role in LTD. Here we demonstrate that the effects of PSD-95 on AMPAR-mediated synaptic responses and LTD can be dissociated. Our findings suggest that N-terminal-domain-mediated dimerization is important for PSD-95's effect on basal synaptic AMPAR function, whereas the C-terminal SH(3)-GK domains are also necessary for localizing PSD-95 to synapses. We identify PSD-95 point mutants (Q15A, E17R) that maintain PSD-95's influence on basal AMPAR synaptic responses yet block LTD. These point mutants increase the proteolysis of PSD-95 within its N-terminal domain, resulting in a C-terminal fragment that functions as a dominant negative likely by scavenging critical signaling proteins required for LTD. Thus, the C-terminal portion of PSD-95 serves a dual function. It is required to localize PSD-95 at synapses and as a scaffold for signaling proteins that are required for LTD.
Figures


References
-
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. - PubMed
-
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
-
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nature. Neurosci. 1999;2:454–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

