Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders
- PMID: 18215785
- PMCID: PMC2259225
- DOI: 10.1016/j.bbmt.2007.11.012
Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders
Abstract
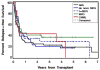
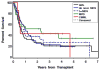
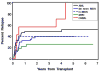
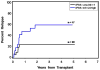
Allogeneic hematopoietic cell transplantation (HCT) is the only curative strategy for patients with myelodysplastic syndrome (MDS) and myeloproliferative disorders (MPD). We report the results of 148 patients (median age = 59 years old) with de novo MDS (n = 40), acute myelogenous leukemia (AML) after antecedent MDS/MPD (n = 49), treatment-related MDS (t-MDS) (n = 25), MPD (n = 27), and chronic myelomonocytic leukemia (CMML) (n = 7) who underwent allogeneic HCT using a conditioning regimen of low-dose total body irradiation (TBI) alone (200 cGy) on day 0 (n = 5) or with the addition of fludarabine (Flu) 30 mg/m(2)/day on days -4 to -2 (n = 143). Postgrafting immunosuppression consisted of cyclosporine and mycophenolate mofetil (MMF). Seventy-five patients (51%) received an allograft from a matched related donor (MRD), and 73 patients (49%) were recipients of unrelated donor (URD) grafts. There was no significant difference in the incidence of acute (gr II-IV) and chronic extensive graft-versus-host disease (aGVHD, cGVHD) between the recipients of related and unrelated donor grafts. By day +28, 75% of patients demonstrated mixed T cell chimerism. Graft rejection was seen in 15% of patients. With a median follow-up of 47 (range: 6-89) months, the 3-year relapse-free survival (RFS) and overall survival (OS) are both 27% for all patients, with a relapse incidence of 41%. The 3-year RFS for the patients with de novo MDS, AML after antecedent MDS/MPD, t-MDS, MPD, and CMML were 22%, 20%, 29%, 37%, and 43%, respectively, and the 3-year OS was 20%, 23%, 27%, 43%, and 43%, respectively. The 3-year nonrelapse mortality (NRM) was 32%. Factors associated with a lower risk of relapse were the development of extensive cGVHD and having a low risk or intermediate-1 risk International Prognostic Score for the de novo MDS patients. Nonmyeloablative HCT confers remissions in patients who otherwise were not eligible for conventional HCT but for whom relapse is the leading cause of treatment failure.
Figures

Similar articles
-
Addition of 10-Day Decitabine to Fludarabine/Total Body Irradiation Conditioning is Feasible and Induces Tumor-Associated Antigen-Specific T Cell Responses.Biol Blood Marrow Transplant. 2016 Jun;22(6):1000-1008. doi: 10.1016/j.bbmt.2016.02.003. Epub 2016 Feb 6. Biol Blood Marrow Transplant. 2016. PMID: 26860635
-
Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia.Biol Blood Marrow Transplant. 2014 Apr;20(4):549-55. doi: 10.1016/j.bbmt.2014.01.009. Epub 2014 Jan 16. Biol Blood Marrow Transplant. 2014. PMID: 24440648 Free PMC article. Clinical Trial.
-
Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden.Biol Blood Marrow Transplant. 2009 Jan;15(1):30-8. doi: 10.1016/j.bbmt.2008.10.012. Biol Blood Marrow Transplant. 2009. PMID: 19135940
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Enhanced Survival of Chronic Myelomonocytic Leukemia-Dysplastic over Proliferative Subtype after Allogeneic Hematopoietic Cell Transplant: A Tertiary Center Experience and Literature Review.Acta Haematol. 2025;148(2):198-207. doi: 10.1159/000539880. Epub 2024 Jun 26. Acta Haematol. 2025. PMID: 38934131 Review.
Cited by
-
Minimal Identifiable Disease and the Role of Conditioning Intensity in Hematopoietic Cell Transplantation for Myelodysplastic Syndrome and Acute Myelogenous Leukemia Evolving from Myelodysplastic Syndrome.Biol Blood Marrow Transplant. 2016 Jul;22(7):1227-1233. doi: 10.1016/j.bbmt.2016.03.029. Epub 2016 Apr 6. Biol Blood Marrow Transplant. 2016. PMID: 27064057 Free PMC article.
-
Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning.Biol Blood Marrow Transplant. 2012 Nov;18(11):1727-33. doi: 10.1016/j.bbmt.2012.06.014. Epub 2012 Jul 2. Biol Blood Marrow Transplant. 2012. PMID: 22766220 Free PMC article.
-
Who is fit for allogeneic transplantation?Blood. 2010 Dec 2;116(23):4762-70. doi: 10.1182/blood-2010-07-259358. Epub 2010 Aug 11. Blood. 2010. PMID: 20702782 Free PMC article. Review.
-
Allogeneic hematopoietic cell transplantation for MDS: for whom, when and how?Blood Rev. 2012 Nov;26(6):247-54. doi: 10.1016/j.blre.2012.08.002. Epub 2012 Sep 13. Blood Rev. 2012. PMID: 22981712 Free PMC article. Review.
-
Impact of irradiation and immunosuppressive agents on immune system homeostasis in rhesus macaques.Clin Exp Immunol. 2015 Sep;181(3):491-510. doi: 10.1111/cei.12646. Epub 2015 Jun 29. Clin Exp Immunol. 2015. PMID: 25902927 Free PMC article.
References
-
- de Witte T, Hermans J, Vossen J, et al. Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110:620–630. - PubMed
-
- Sierra J, Perez WS, Rozman C, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100:1997–2004. - PubMed
-
- Deeg HJ, Gooley TA, Flowers ME, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood. 2003;102:3912–3918. - PubMed
-
- Guardiola P, Anderson JE, Bandini G, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Societe Francaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood. 1999;93:2831–2838. - PubMed
-
- Witherspoon RP, Deeg HJ, Storer B, et al. Hematopoietic stem-cell transplantation for treatment-related leukemia or myelodysplasia. J Clin Oncol. 2001;19:2134–2141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

