The design and implementation of A5146, a prospective trial assessing the utility of therapeutic drug monitoring using an inhibitory quotient in antiretroviral-experienced HIV-infected patients
- PMID: 18215983
- PMCID: PMC2821072
- DOI: 10.1310/hct0901-61
The design and implementation of A5146, a prospective trial assessing the utility of therapeutic drug monitoring using an inhibitory quotient in antiretroviral-experienced HIV-infected patients
Abstract
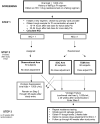
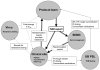
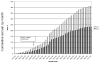
The AIDS Clinical Trials Group designed and implemented a prospective, randomized, strategy trial in antiretroviral-experienced, HIV-infected patients to evaluate the virologic impact of protease inhibitor dose escalation in response to therapeutic drug monitoring (TDM) with an inhibitory quotient, which integrates both drug exposure and viral drug resistance. In the process of developing this clinical trial, several unique challenges were identified that required innovative solutions. The major challenge was the need to integrate resistance testing, pharmacokinetic data, medication adherence, toxicity data, clinical assessments, randomization assignment, and protocol-specified clinical management in a way that could be utilized in real time by the protocol team, communicated promptly to the clinical sites, and transmitted accurately to the study database. In addition, the protocol team had to address the relative lack of commercially available TDM laboratories in the United States that were experienced in antiretroviral drug assays and a lack of familiarity with the principles of pharmacokinetic monitoring at participating clinical sites. This article outlines the rationale for the design of this strategy trial, specific barriers to implementation that were identified, and solutions that were developed with the hope that these experiences will facilitate the design and conduct of future trials of TDM.
Figures

References
-
- Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004 Apr 29;350(18):1850–1861. - PubMed
-
- Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002 Jun 27;346(26):2039–2046. - PubMed
-
- Lalezari JP, Henry K, O'Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003 May 29;348(22):2175–2185. - PubMed
-
- Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003 May 29;348(22):2186–2195. - PubMed
-
- Katlama C, Esposito R, Gatell JM, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS. 2007 Feb 19;21(4):395–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01-RR00044/RR/NCRR NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- N01-AI-38858/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- U01 AI-069472/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- U01-AI-069511/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- N01-AI-68636/AI/NIAID NIH HHS/United States
- U01 AI-38855/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI-068634/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
