RPE65 from cone-dominant chicken is a more efficient isomerohydrolase compared with that from rod-dominant species
- PMID: 18216020
- PMCID: PMC2276383
- DOI: 10.1074/jbc.M703654200
RPE65 from cone-dominant chicken is a more efficient isomerohydrolase compared with that from rod-dominant species
Abstract
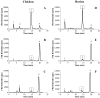
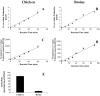
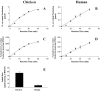
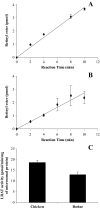
Cones recover their photosensitivity faster than rods after bleaching. It has been suggested that a higher rate regeneration of 11-cis-retinal, the chromophore for visual pigments, is required for cones to continuously function under bright light conditions. RPE65 is the isomerohydrolase catalyzing a key step in regeneration of 11-cis-retinal. The present study investigated whether RPE65 in a cone-dominant species is more efficient in its enzymatic activity than that from roddominant species. In vitro isomerohydrolase activity assay showed that isomerohydrolase activity in the chicken retinal pigment epithelium (RPE) was 11.7-fold higher than in the bovine RPE, after normalization by RPE65 protein levels. Similar to that of human and bovine, the isomerohydrolase activity in chicken RPE was blocked by two specific inhibitors of lecithin retinal acyltransferase, indicating that chicken RPE65 also uses all-trans-retinyl ester as the direct substrate. To exclude the possibility that the higher isomerohydrolase activity in the chicken RPE could arise from another unknown isomerohydrolase, we expressed chicken and human RPE65 using the adenovirus system in a stable cell line expressing lecithin retinal acyltransferase. Under the same conditions, isomerohydrolase activity of recombinant chicken RPE65 was 7.7-fold higher than that of recombinant human RPE65, after normalization by RPE65 levels. This study demonstrates that RPE65 from the cone-dominant chicken RPE possesses significantly higher specific retinol isomerohydrolase activity, when compared with RPE65 from rod-dominant species, consistent with the faster regeneration rates of visual pigments in cone-dominant retinas.
Figures


Similar articles
-
Regeneration of photopigment is enhanced in mouse cone photoreceptors expressing RPE65 protein.J Neurosci. 2011 Jul 13;31(28):10403-11. doi: 10.1523/JNEUROSCI.0182-11.2011. J Neurosci. 2011. PMID: 21753017 Free PMC article.
-
An alternative isomerohydrolase in the retinal Müller cells of a cone-dominant species.FEBS J. 2011 Aug;278(16):2913-26. doi: 10.1111/j.1742-4658.2011.08216.x. Epub 2011 Jul 1. FEBS J. 2011. PMID: 21676174 Free PMC article.
-
RPE65 is the isomerohydrolase in the retinoid visual cycle.Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12413-8. doi: 10.1073/pnas.0503460102. Epub 2005 Aug 22. Proc Natl Acad Sci U S A. 2005. PMID: 16116091 Free PMC article.
-
Retinal pigment epithelium 65 kDa protein (RPE65): An update.Prog Retin Eye Res. 2022 May;88:101013. doi: 10.1016/j.preteyeres.2021.101013. Epub 2021 Oct 2. Prog Retin Eye Res. 2022. PMID: 34607013 Free PMC article. Review.
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
Cited by
-
Retinoid isomerase inhibitors impair but do not block mammalian cone photoreceptor function.J Gen Physiol. 2018 Apr 2;150(4):571-590. doi: 10.1085/jgp.201711815. Epub 2018 Mar 2. J Gen Physiol. 2018. PMID: 29500274 Free PMC article.
-
Identification of the key residues determining the product specificity of isomerohydrolase.Biochemistry. 2012 May 22;51(20):4217-25. doi: 10.1021/bi300144n. Epub 2012 May 14. Biochemistry. 2012. PMID: 22512451 Free PMC article.
-
RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):10882-10887. doi: 10.1073/pnas.1706332114. Epub 2017 Sep 5. Proc Natl Acad Sci U S A. 2017. PMID: 28874556 Free PMC article.
-
Leukemia inhibitory factor coordinates the down-regulation of the visual cycle in the retina and retinal-pigmented epithelium.J Biol Chem. 2012 Jul 13;287(29):24092-102. doi: 10.1074/jbc.M112.378240. Epub 2012 May 29. J Biol Chem. 2012. PMID: 22645143 Free PMC article.
-
Molecular components affecting ocular carotenoid and retinoid homeostasis.Prog Retin Eye Res. 2021 Jan;80:100864. doi: 10.1016/j.preteyeres.2020.100864. Epub 2020 Apr 25. Prog Retin Eye Res. 2021. PMID: 32339666 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

