Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens
- PMID: 18216021
- PMCID: PMC2442294
- DOI: 10.1074/jbc.M709545200
Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens
Abstract
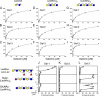
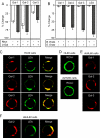
Human galectins have functionally divergent roles, although most of the members of the galectin family bind weakly to the simple disaccharide lactose (Galbeta1-4Glc). To assess the specificity of galectin-glycan interactions in more detail, we explored the binding of several important galectins (Gal-1, Gal-2, and Gal-3) using a dose-response approach toward a glycan microarray containing hundreds of structurally diverse glycans, and we compared these results to binding determinants on cells. All three galectins exhibited differences in glycan binding characteristics. On both the microarray and on cells, Gal-2 and Gal-3 exhibited higher binding than Gal-1 to fucose-containing A and B blood group antigens. Gal-2 exhibited significantly reduced binding to all sialylated glycans, whereas Gal-1 bound alpha2-3- but not alpha2-6-sialylated glycans, and Gal-3 bound to some glycans terminating in either alpha2-3- or alpha2-6-sialic acid. The effects of sialylation on Gal-1, Gal-2, and Gal-3 binding to cells also reflected differences in cellular sensitivity to Gal-1-, Gal-2-, and Gal-3-induced phosphatidylserine exposure. Each galectin exhibited higher binding for glycans with poly-N-acetyllactosamine (poly(LacNAc)) sequences (Galbeta1-4GlcNAc)(n) when compared with N-acetyllactosamine (LacNAc) glycans (Galbeta1-4GlcNAc). However, only Gal-3 bound internal LacNAc within poly(LacNAc). These results demonstrate that each of these galectins mechanistically differ in their binding to glycans on the microarrays and that these differences are reflected in the determinants required for cell binding and signaling. The specific glycan recognition by each galectin underscores the basis for differences in their biological activities.
Figures








References
-
- Camby, I., Le Mercier, M., Lefranc, F., and Kiss, R. (2006) Glycobiology 16 R137-R157 - PubMed
-
- Dumic, J., Dabelic, S., and Flogel, M. (2006) Biochim. Biophys. Acta 1760 616-635 - PubMed
-
- Leffler, H., Carlsson, S., Hedlund, M., Qian, Y., and Poirier, F. (2004) Glycoconj. J. 19 433-440 - PubMed
-
- Rubinstein, N., Ilarregui, J. M., Toscano, M. A., and Rabinovich, G. A. (2004) Tissue Antigens 64 1-12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

