Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation
- PMID: 18216022
- PMCID: PMC2417166
- DOI: 10.1074/jbc.M706105200
Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation
Abstract
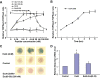
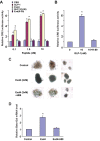
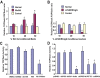
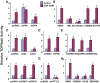
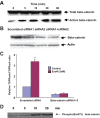
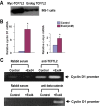
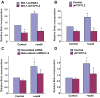
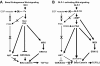
The insulinotropic hormone GLP-1 (glucagon-like peptide-1) is a new therapeutic agent that preserves or restores pancreatic beta cell mass. We report that GLP-1 and its agonist, exendin-4 (Exd4), induce Wnt signaling in pancreatic beta cells, both isolated islets, and in INS-1 cells. Basal and GLP-1 agonist-induced proliferation of beta cells requires active Wnt signaling. Cyclin D1 and c-Myc, determinants of cell proliferation, are up-regulated by Exd4. Basal endogenous Wnt signaling activity depends on Wnt frizzled receptors and the protein kinases Akt and GSK3beta but not cAMP-dependent protein kinase. In contrast, GLP-1 agonists enhance Wnt signaling via GLP-1 receptor-mediated activation of Akt and beta cell independent of GSK3beta. Inhibition of Wnt signaling by small interfering RNAs to beta-catenin or a dominant-negative TCF7L2 decreases both basal and Exd4-induced beta cell proliferation. Wnt signaling appears to mediate GLP-1-induced beta cell proliferation raising possibilities for novel treatments of diabetes.
Figures
Similar articles
-
Exendin-4 induction of cyclin D1 expression in INS-1 beta-cells: involvement of cAMP-responsive element.J Endocrinol. 2006 Mar;188(3):623-33. doi: 10.1677/joe.1.06480. J Endocrinol. 2006. PMID: 16522741
-
The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells.Breast Cancer Res Treat. 2012 Apr;132(2):449-61. doi: 10.1007/s10549-011-1585-0. Epub 2011 Jun 3. Breast Cancer Res Treat. 2012. PMID: 21638053
-
Exendin-4 upregulates the expression of Wnt-4, a novel regulator of pancreatic β-cell proliferation.Am J Physiol Endocrinol Metab. 2011 Nov;301(5):E864-72. doi: 10.1152/ajpendo.00144.2011. Epub 2011 Jul 19. Am J Physiol Endocrinol Metab. 2011. PMID: 21771967
-
New insight into the mechanisms underlying the function of the incretin hormone glucagon-like peptide-1 in pancreatic β-cells: the involvement of the Wnt signaling pathway effector β-catenin.Islets. 2012 Nov-Dec;4(6):359-65. doi: 10.4161/isl.23345. Epub 2012 Nov 1. Islets. 2012. PMID: 23314611 Free PMC article. Review.
-
Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells.Metabolism. 2014 Jan;63(1):9-19. doi: 10.1016/j.metabol.2013.09.010. Epub 2013 Oct 17. Metabolism. 2014. PMID: 24140094 Review.
Cited by
-
An olive-derived elenolic acid stimulates hormone release from L-cells and exerts potent beneficial metabolic effects in obese diabetic mice.Front Nutr. 2022 Nov 1;9:1051452. doi: 10.3389/fnut.2022.1051452. eCollection 2022. Front Nutr. 2022. PMID: 36386896 Free PMC article.
-
Irisin and Incretin Hormones: Similarities, Differences, and Implications in Type 2 Diabetes and Obesity.Biomolecules. 2021 Feb 15;11(2):286. doi: 10.3390/biom11020286. Biomolecules. 2021. PMID: 33671882 Free PMC article. Review.
-
Role of pancreatic transcription factors in maintenance of mature β-cell function.Int J Mol Sci. 2015 Mar 18;16(3):6281-97. doi: 10.3390/ijms16036281. Int J Mol Sci. 2015. PMID: 25794287 Free PMC article. Review.
-
Glucagon-Like Peptide-1: Actions and Influence on Pancreatic Hormone Function.Compr Physiol. 2020 Mar 12;10(2):577-595. doi: 10.1002/cphy.c190025. Compr Physiol. 2020. PMID: 32163198 Free PMC article. Review.
-
Inventing Liraglutide, a Glucagon-Like Peptide-1 Analogue, for the Treatment of Diabetes and Obesity.ACS Pharmacol Transl Sci. 2019 Aug 20;2(6):468-484. doi: 10.1021/acsptsci.9b00048. eCollection 2019 Dec 13. ACS Pharmacol Transl Sci. 2019. PMID: 32259078 Free PMC article.
References
-
- Holst, J. J. (2004) Expert Opin. Emerg. Drugs 9 155-166 - PubMed
-
- Drucker, D. J., and Nauck, M. A. (2006) Lancet 368 1696-1705 - PubMed
-
- Kieffer, T. J., and Habener, J. F. (1999) Endocr. Rev. 20 876-913 - PubMed
-
- Egan, J. M., Bulotta, A., Hui, H., and Perfetti, R. (2003) Diabetes Metab. Res. Rev. 19 115-123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

