Retrograde signaling onto Ret during motor nerve terminal maturation
- PMID: 18216204
- PMCID: PMC6671002
- DOI: 10.1523/JNEUROSCI.4489-07.2008
Retrograde signaling onto Ret during motor nerve terminal maturation
Abstract
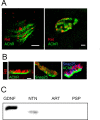
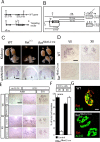
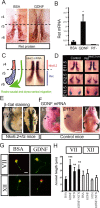
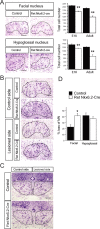
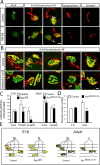
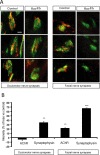
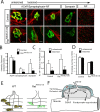
Establishment of the neuromuscular synapse requires bidirectional signaling between the nerve and muscle. Although much is known on nerve-released signals onto the muscle, less is known of signals important for presynaptic maturation of the nerve terminal. Our results suggest that the Ret tyrosine kinase receptor transmits a signal in motor neuron synapses that contribute to motor neuron survival and synapse maturation at postnatal stages. Ret is localized specifically to the presynaptic membrane with its ligands, GDNF (glial cell line-derived neurotrophic factor)/NTN (neurturin), expressed in skeletal muscle tissue. Lack of Ret conditionally in cranial motor neurons results in a developmental deficit of maturation and specialization of presynaptic neuromuscular terminals. Regeneration of Ret-deficient adult hypoglossal motor neurons is unperturbed, but despite contact with the unaffected postsynaptic specializations, presynaptic axon terminal maturation is severely compromised in the absence of Ret signaling. Thus, Ret transmits a signal in motor nerve terminals that participate in the organization and maturation of presynaptic specializations during development and during regeneration in the adult.
Figures
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Baudet C, Mikaels A, Westphal H, Johansen J, Johansen TE, Ernfors P. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development. 2000;127:4335–4344. - PubMed
-
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
