Efficient coupling of catalysis and dynamics in the E1 component of Escherichia coli pyruvate dehydrogenase multienzyme complex
- PMID: 18216265
- PMCID: PMC2234108
- DOI: 10.1073/pnas.0709328105
Efficient coupling of catalysis and dynamics in the E1 component of Escherichia coli pyruvate dehydrogenase multienzyme complex
Abstract
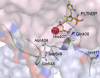
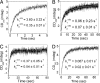
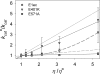
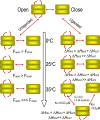
Protein motions are ubiquitous and are intrinsically coupled to catalysis. Their specific roles, however, remain largely elusive. Dynamic loops at the active center of the E1 component of Escherichia coli pyruvate dehydrogenase multienzyme complex are essential for several catalytic functions starting from a predecarboxylation event and culminating in transfer of the acetyl moiety to the E2 component. Monitoring the kinetics of E1 and its loop variants at various solution viscosities, we show that the rate of a chemical step is modulated by loop dynamics. A cysteine-free E1 construct was site-specifically labeled on the inner loop (residues 401-413), and the EPR nitroxide label revealed ligand-induced conformational dynamics of the loop and a slow "open <--> close" conformational equilibrium in the unliganded state. An (19)F NMR label placed at the same residue revealed motion on the millisecond-second time scale and suggested a quantitative correlation of E1 catalysis and loop dynamics for the 200,000-Da protein. Thermodynamic studies revealed that these motions may promote covalent addition of substrate to the enzyme-bound thiamin diphosphate by reducing the free energy of activation. Furthermore, the global dynamics of E1 presumably regulate and streamline the catalytic steps of the overall complex by inducing an entirely entropic (nonmechanical) negative cooperativity with respect to substrate binding at higher temperatures. Our results are consistent with, and reinforce the hypothesis of, coupling of catalysis and regulation with enzyme dynamics and suggest the mechanism by which it is achieved in a key branchpoint enzyme in sugar metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nuclear magnetic resonance approaches in the study of 2-oxo acid dehydrogenase multienzyme complexes--a literature review.Molecules. 2013 Sep 26;18(10):11873-903. doi: 10.3390/molecules181011873. Molecules. 2013. PMID: 24077172 Free PMC article. Review.
-
Multiple roles of mobile active center loops in the E1 component of the Escherichia coli pyruvate dehydrogenase complex - Linkage of protein dynamics to catalysis.J Mol Catal B Enzym. 2009 Nov 1;61(1-2):14-22. doi: 10.1016/j.molcatb.2009.04.008. J Mol Catal B Enzym. 2009. PMID: 20160956 Free PMC article.
-
Conformational ensemble modulates cooperativity in the rate-determining catalytic step in the E1 component of the Escherichia coli pyruvate dehydrogenase multienzyme complex.J Biol Chem. 2009 Nov 27;284(48):33122-9. doi: 10.1074/jbc.M109.065508. Epub 2009 Sep 29. J Biol Chem. 2009. PMID: 19801660 Free PMC article.
-
Phosphorylation of serine 264 impedes active site accessibility in the E1 component of the human pyruvate dehydrogenase multienzyme complex.Biochemistry. 2007 May 29;46(21):6277-87. doi: 10.1021/bi700083z. Epub 2007 May 3. Biochemistry. 2007. PMID: 17474719
-
Enzyme dynamics from NMR spectroscopy.Acc Chem Res. 2015 Feb 17;48(2):457-65. doi: 10.1021/ar500340a. Epub 2015 Jan 9. Acc Chem Res. 2015. PMID: 25574774 Free PMC article. Review.
Cited by
-
Experimental observation of thiamin diphosphate-bound intermediates on enzymes and mechanistic information derived from these observations.Bioorg Chem. 2005 Jun;33(3):190-215. doi: 10.1016/j.bioorg.2005.02.001. Epub 2005 Apr 1. Bioorg Chem. 2005. PMID: 15888311 Free PMC article. Review.
-
Mix-and-inject XFEL crystallography reveals gated conformational dynamics during enzyme catalysis.Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25634-25640. doi: 10.1073/pnas.1901864116. Epub 2019 Dec 4. Proc Natl Acad Sci U S A. 2019. PMID: 31801874 Free PMC article.
-
Coupled motions in enzyme catalysis.Curr Opin Chem Biol. 2010 Oct;14(5):644-51. doi: 10.1016/j.cbpa.2010.07.020. Epub 2010 Aug 20. Curr Opin Chem Biol. 2010. PMID: 20729130 Free PMC article. Review.
-
Nuclear magnetic resonance approaches in the study of 2-oxo acid dehydrogenase multienzyme complexes--a literature review.Molecules. 2013 Sep 26;18(10):11873-903. doi: 10.3390/molecules181011873. Molecules. 2013. PMID: 24077172 Free PMC article. Review.
-
Multiple roles of mobile active center loops in the E1 component of the Escherichia coli pyruvate dehydrogenase complex - Linkage of protein dynamics to catalysis.J Mol Catal B Enzym. 2009 Nov 1;61(1-2):14-22. doi: 10.1016/j.molcatb.2009.04.008. J Mol Catal B Enzym. 2009. PMID: 20160956 Free PMC article.
References
-
- Koike M, Reed LJ, Carroll WR. J Biol Chem. 1960;235:1924–1930. - PubMed
-
- Arjunan P, Nemeria N, Brunskill A, Chandrasekhar K, Sax M, Yan Y, Jordan F, Guest JR, Furey W. Biochemistry. 2002;41:5213–5221. - PubMed
-
- Arjunan P, Sax M, Brunskill A, Chandrasekhar K, Nemeria N, Zhang S, Jordan F, Furey W. J Biol Chem. 2006;281:15296–15303. - PubMed
-
- Kale S, Arjunan P, Furey W, Jordan F. J Biol Chem. 2007;282:28106–28116. - PubMed
-
- Kern D, Eisenmesser EZ, Wolf-Watz M. In: Methods in Enzymology. James TL, editor. New York: Academic; 2005. pp. 507–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

