Signal processing by its coil zipper domain activates IKK gamma
- PMID: 18216269
- PMCID: PMC2234129
- DOI: 10.1073/pnas.0706552105
Signal processing by its coil zipper domain activates IKK gamma
Abstract
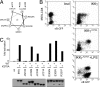
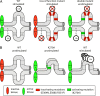
NF-kappaB activation occurs upon degradation of its inhibitor I-kappaB and requires prior phosphorylation of the inhibitor by I-kappaB kinase (IKK). Activity of IKK is governed by its noncatalytic subunit IKKgamma. Signaling defects due to missense mutations in IKKgamma have been correlated to its inability to either become ubiquitylated or bind ubiquitin noncovalently. Because the relative contribution of these events to signaling had remained unknown, we have studied mutations in the coil-zipper (CoZi) domain of IKKgamma that either impair signaling or cause constitutive NF-kappaB activity. Certain signaling-deficient alleles neither bound ubiquitin nor were they ubiquitylated by TRAF6. Introducing an activating mutation into those signaling-impaired alleles restored their ubiquitylation and created mutants constitutively activating NF-kappaB without repairing the ubiquitin-binding defect. Constitutive activity therefore arises downstream of ubiquitin binding but upstream of ubiquitylation. Such constitutive activity reveals a signal-processing function for IKKgamma beyond that of a mere ubiquitin-binding adaptor. We propose that this signal processing may involve homophilic CoZi interactions as suggested by the enhanced affinity of CoZi domains from constitutively active IKKgamma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

