Coordinated regulation of Arabidopsis thaliana development by light and gibberellins
- PMID: 18216856
- PMCID: PMC2562044
- DOI: 10.1038/nature06448
Coordinated regulation of Arabidopsis thaliana development by light and gibberellins
Abstract
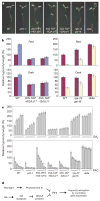
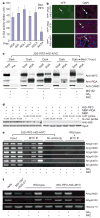
Light and gibberellins (GAs) mediate many essential and partially overlapping plant developmental processes. DELLA proteins are GA-signalling repressors that block GA-induced development. GA induces degradation of DELLA proteins via the ubiquitin/proteasome pathway, but light promotes accumulation of DELLA proteins by reducing GA levels. It was proposed that DELLA proteins restrain plant growth largely through their effect on gene expression. However, the precise mechanism of their function in coordinating GA signalling and gene expression remains unknown. Here we characterize a nuclear protein interaction cascade mediating transduction of GA signals to the activity regulation of a light-responsive transcription factor. In the absence of GA, nuclear-localized DELLA proteins accumulate to higher levels, interact with phytochrome-interacting factor 3 (PIF3, a bHLH-type transcription factor) and prevent PIF3 from binding to its target gene promoters and regulating gene expression, and therefore abrogate PIF3-mediated light control of hypocotyl elongation. In the presence of GA, GID1 proteins (GA receptors) elevate their direct interaction with DELLA proteins in the nucleus, trigger DELLA protein's ubiquitination and proteasome-mediated degradation, and thus release PIF3 from the negative effect of DELLA proteins.
Figures


Similar articles
-
Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis.Plant Physiol. 2011 Jul;156(3):1410-23. doi: 10.1104/pp.111.177741. Epub 2011 May 11. Plant Physiol. 2011. PMID: 21562334 Free PMC article.
-
DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis.Nat Commun. 2016 Jun 10;7:11868. doi: 10.1038/ncomms11868. Nat Commun. 2016. PMID: 27282989 Free PMC article.
-
A molecular framework for light and gibberellin control of cell elongation.Nature. 2008 Jan 24;451(7177):480-4. doi: 10.1038/nature06520. Nature. 2008. PMID: 18216857
-
Molecular biology of gibberellins signaling in higher plants.Int Rev Cell Mol Biol. 2008;268:191-221. doi: 10.1016/S1937-6448(08)00806-X. Int Rev Cell Mol Biol. 2008. PMID: 18703407 Review.
-
Plant hormone signaling lightens up: integrators of light and hormones.Curr Opin Plant Biol. 2010 Oct;13(5):571-7. doi: 10.1016/j.pbi.2010.07.001. Epub 2010 Aug 23. Curr Opin Plant Biol. 2010. PMID: 20739215 Review.
Cited by
-
Manipulation of plant architecture to enhance lignocellulosic biomass.AoB Plants. 2012;2012:pls026. doi: 10.1093/aobpla/pls026. Epub 2012 Oct 13. AoB Plants. 2012. PMID: 23071897 Free PMC article.
-
Wheat genomic study for genetic improvement of traits in China.Sci China Life Sci. 2022 Sep;65(9):1718-1775. doi: 10.1007/s11427-022-2178-7. Epub 2022 Aug 24. Sci China Life Sci. 2022. PMID: 36018491 Review.
-
Brassinosteroid signalling.Development. 2013 Apr;140(8):1615-20. doi: 10.1242/dev.060590. Development. 2013. PMID: 23533170 Free PMC article. Review.
-
Differences and similarities in the photoregulation of gibberellin metabolism between rice and dicots.Plant Signal Behav. 2013 Mar;8(3):e23424. doi: 10.4161/psb.23424. Epub 2013 Jan 18. Plant Signal Behav. 2013. PMID: 23333965 Free PMC article.
-
The ERF11 Transcription Factor Promotes Internode Elongation by Activating Gibberellin Biosynthesis and Signaling.Plant Physiol. 2016 Aug;171(4):2760-70. doi: 10.1104/pp.16.00154. Epub 2016 Jun 2. Plant Physiol. 2016. PMID: 27255484 Free PMC article.
References
-
- Fleet CM, Sun TPA. DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol. 2005;8:77–85. - PubMed
-
- Itoh H, Matsuoka M, Steber CM. A role for the ubiquitin–26S-proteasome pathway in gibberellin signaling. Trends Plant Sci. 2003;8:492–497. - PubMed
-
- Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218:683–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

