High titer autoantibodies to GM-CSF in patients with AML, CML and MDS are associated with active disease
- PMID: 18216869
- PMCID: PMC3403381
- DOI: 10.1038/sj.leu.2405104
High titer autoantibodies to GM-CSF in patients with AML, CML and MDS are associated with active disease
Abstract
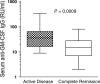
Antibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF) can be induced when GM-CSF is used as an adjuvant to solid tumor vaccination. Neutralizing anti-GM-CSF IgG has been associated with pulmonary alveolar proteinosis (PAP), and secondary PAP has been linked to myeloid leukemia. We studied 69 patients with acute myeloid leukemia, chronic myeloid leukemia and myelodysplastic syndrome, including 19 patients who received GM-CSF with peptide antigen and incomplete Freund's adjuvant in a vaccine trial for the presence or induction of anti-GM-CSF antibodies. Anti-GM-CSF IgG were present in 36 (52%) patients with myeloid leukemia compared to only 1 of 33 (3%) healthy subjects (P=0.008) and in none of 6 patients with lymphoid leukemia (P=0.0001). Antibody titers were unaffected by vaccination. Anti-GM-CSF IgA and IgM were found in 33 and 20% of patients, respectively; IgA from two patients neutralized GM-CSF. Strikingly, while anti-GM-CSF IgG titers were higher in patients with active disease (n=52) versus those in complete remission (n=14, P=0.0009), GM-CSF expression was not increased in either group. These data are first to show that anti-GM-CSF antibodies of multiple isotypes are present in patients with active myeloid leukemia without PAP and may be useful markers of disease activity.
Figures
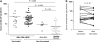


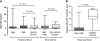
Similar articles
-
Anti-GM-CSF autoantibodies in myeloid leukemias.Best Pract Res Clin Haematol. 2025 Mar;38(1):101611. doi: 10.1016/j.beha.2025.101611. Epub 2025 Mar 12. Best Pract Res Clin Haematol. 2025. PMID: 40274339 Review.
-
Disseminated Cryptococcosis Due to Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Absence of Pulmonary Alveolar Proteinosis.J Clin Immunol. 2017 Feb;37(2):143-152. doi: 10.1007/s10875-016-0364-4. Epub 2016 Dec 24. J Clin Immunol. 2017. PMID: 28013480
-
The Pathogenic Role of Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Nocardiosis with the Central Nervous System Involvement.J Clin Immunol. 2024 Aug 12;44(8):176. doi: 10.1007/s10875-024-01775-w. J Clin Immunol. 2024. PMID: 39133333
-
Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis.Am J Respir Cell Mol Biol. 2002 Oct;27(4):481-6. doi: 10.1165/rcmb.2002-0023OC. Am J Respir Cell Mol Biol. 2002. PMID: 12356582
-
Secondary pulmonary alveolar proteinosis: a single-center retrospective study (a case series and literature review).BMC Pulm Med. 2018 Jan 25;18(1):15. doi: 10.1186/s12890-018-0590-z. BMC Pulm Med. 2018. PMID: 29368649 Free PMC article. Review.
Cited by
-
SP-D counteracts GM-CSF-mediated increase of granuloma formation by alveolar macrophages in lysinuric protein intolerance.Orphanet J Rare Dis. 2009 Dec 23;4:29. doi: 10.1186/1750-1172-4-29. Orphanet J Rare Dis. 2009. PMID: 20030831 Free PMC article.
-
Pulmonary alveolar proteinosis.Eur Respir Rev. 2011 Jun;20(120):98-107. doi: 10.1183/09059180.00001311. Eur Respir Rev. 2011. PMID: 21632797 Free PMC article. Review.
-
An updated review on phenocopies of primary immunodeficiency diseases.Genes Dis. 2019 Sep 24;7(1):12-25. doi: 10.1016/j.gendis.2019.09.007. eCollection 2020 Mar. Genes Dis. 2019. PMID: 32181272 Free PMC article. Review.
-
High Expression of ENO1 and Low Levels of Circulating Anti-ENO1 Autoantibodies in Patients with Myelodysplastic Neoplasms and Acute Myeloid Leukaemia.Cancers (Basel). 2024 Feb 22;16(5):884. doi: 10.3390/cancers16050884. Cancers (Basel). 2024. PMID: 38473245 Free PMC article.
-
Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis.Nat Commun. 2015 Jun 16;6:7375. doi: 10.1038/ncomms8375. Nat Commun. 2015. PMID: 26077231 Free PMC article.
References
-
- Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. - PubMed
-
- Young DC, Griffin JD. Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood. 1986;68:1178–1181. - PubMed
-
- Weisbart RH, Golde DW, Clark SC, Wong GG, Gasson JC. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314:361–363. - PubMed
-
- Metcalf D. Molecular control of granulocyte and macrophage production. Prog Clin Biol Res. 1985;191:323–337. - PubMed
-
- Vasilijic S, Colic M, Vucevic D. Granulocyte-macrophage colony stimulating factor is an anti-apoptotic cytokine for thymic dendritic cells and a significant modulator of their accessory function. Immunol Lett. 2003;86:99–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

