Protein quality control in the early secretory pathway
- PMID: 18216874
- PMCID: PMC2234347
- DOI: 10.1038/sj.emboj.7601974
Protein quality control in the early secretory pathway
Abstract
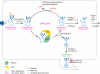
Eukaryotic cells are able to discriminate between native and non-native polypeptides, selectively transporting the former to their final destinations. Secretory proteins are scrutinized at the endoplasmic reticulum (ER)-Golgi interface. Recent findings reveal novel features of the underlying molecular mechanisms, with several chaperone networks cooperating in assisting the maturation of complex proteins and being selectively induced to match changing synthetic demands. 'Public' and 'private' chaperones, some of which enriched in specializes subregions, operate for most or selected substrates, respectively. Moreover, sequential checkpoints are distributed along the early secretory pathway, allowing efficiency and fidelity in protein secretion.
Figures


References
-
- Ahluwalia N, Bergeron JJ, Wada I, Degen E, Williams DB (1992) The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J Biol Chem 267: 10914–10918 - PubMed
-
- Anfinsen CB, Scheraga HA (1975) Experimental and theoretical aspects of protein folding. Adv Protein Chem 29: 205–300 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

