The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens
- PMID: 18218096
- PMCID: PMC2246119
- DOI: 10.1186/1471-2121-9-4
The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens
Abstract
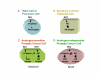
Background: A crucial event in Prostate Cancer progression is the conversion from a hormone-sensitive to a hormone-refractory disease state. Correlating with this transition, androgen receptor (AR) amplification and mutations are often observed in patients failing hormonal ablation therapies. beta-Catenin, an essential component of the canonical Wnt signaling pathway, was shown to be a coactivator of the AR signaling in the presence of androgens. However, it is not yet clear what effect the increased levels of the AR could have on the Wnt signaling pathway in these hormone-refractory prostate cells.
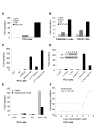
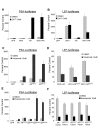
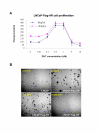
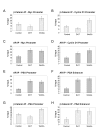
Results: Transient transfections of several human prostate cancer cell lines with the AR and multiple components of the Wnt signaling pathway demonstrate that the AR overexpression can potentiate the transcriptional activities of Wnt/beta-Catenin signaling. In addition, the simultaneous activation of the Wnt signaling pathway and overexpression of the AR promote prostate cancer cell growth and transformation at castration levels of androgens. Interestingly, the presence of physiological levels of androgen or other AR agonists inhibits these effects. These observations are consistent with the nuclear co-localization of the AR and beta-Catenin shown by immunohistochemistry in human prostate cancer samples. Furthermore, chromatin immunoprecipitation assays showed that Wnt3A can recruit the AR to the promoter regions of Myc and Cyclin D1, which are well-characterized downstream targets of the Wnt signalling pathway. The same assays demonstrated that the AR and beta-Catenin can be recruited to the promoter and enhancer regions of a known AR target gene PSA upon Wnt signaling. These results suggest that the AR is promoting Wnt signaling at the chromatin level.
Conclusion: Our findings suggest that the AR signaling through the Wnt/beta-Catenin pathway should be added to the well established functional interactions between both pathways. Moreover, our data show that via this interaction the AR could promote prostate cell malignancy in a ligand-independent manner.
Figures


References
-
- Koivisto P, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–9. - PubMed
-
- Linja MJ, et al. Amplification and Overexpression of Androgen Receptor Gene in Hormone-Refractory Prostate Cancer. Cancer Res. 2001;61:3550–3555. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

