Diverse antidepressants increase CDP-diacylglycerol production and phosphatidylinositide resynthesis in depression-relevant regions of the rat brain
- PMID: 18218113
- PMCID: PMC2245968
- DOI: 10.1186/1471-2202-9-12
Diverse antidepressants increase CDP-diacylglycerol production and phosphatidylinositide resynthesis in depression-relevant regions of the rat brain
Abstract
Background: Major depression is a serious mood disorder affecting millions of adults and children worldwide. While the etiopathology of depression remains obscure, antidepressant medications increase synaptic levels of monoamine neurotransmitters in brain regions associated with the disease. Monoamine transmitters activate multiple signaling cascades some of which have been investigated as potential mediators of depression or antidepressant drug action. However, the diacylglycerol arm of phosphoinositide signaling cascades has not been systematically investigated, even though downstream targets of this cascade have been implicated in depression. With the ultimate goal of uncovering the primary postsynaptic actions that may initiate cellular antidepressive signaling, we have examined the antidepressant-induced production of CDP-diacylglycerol which is both a product of diacylglycerol phosphorylation and a precursor for the synthesis of physiologically critical glycerophospholipids such as the phosphatidylinositides. For this, drug effects on [3H]cytidine-labeled CDP-diacylglycerol and [3H]inositol-labeled phosphatidylinositides were measured in response to the tricyclics desipramine and imipramine, the selective serotonin reuptake inhibitors fluoxetine and paroxetine, the atypical antidepressants maprotiline and nomifensine, and several monoamine oxidase inhibitors.
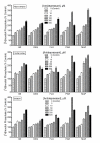
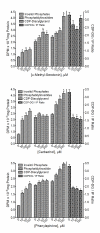
Results: Multiple compounds from each antidepressant category significantly stimulated [3H]CDP-diacylglycerol accumulation in cerebrocortical, hippocampal, and striatal tissues, and also enhanced the resynthesis of inositol phospholipids. Conversely, various antipsychotics, anxiolytics, and non-antidepressant psychotropic agents failed to significantly induce CDP-diacylglycerol or phosphoinositide synthesis. Drug-induced CDP-diacylglycerol accumulation was independent of lithium and only partially dependent on phosphoinositide hydrolysis, thus indicating that antidepressants can mobilize CDP-diacylglycerol from additional pools lying outside of the inositol cycle. Further, unlike direct serotonergic, muscarinic, or alpha-adrenergic agonists that elicited comparable or lower effects on CDP-diacylglycerol versus inositol phosphates, the antidepressants dose-dependently induced significantly greater accumulations of CDP-diacylglycerol.
Conclusion: Chemically divergent antidepressant agents commonly and significantly enhanced the accumulation of CDP-diacylglycerol. The latter is not only a derived product of phosphoinositide hydrolysis but is also a crucial intermediate in the biosynthesis of several signaling substrates. Hence, altered CDP-diacylglycerol signaling might be implicated in the pathophysiology of depression or the mechanism of action of diverse antidepressant medications.
Figures








Similar articles
-
Antidepressant stimulation of CDP-diacylglycerol synthesis does not require monoamine reuptake inhibition.BMC Neurosci. 2010 Jan 27;11:10. doi: 10.1186/1471-2202-11-10. BMC Neurosci. 2010. PMID: 20105322 Free PMC article.
-
Tandem regulation of phosphoinositide signaling and acute behavioral effects induced by antidepressant agents in rats.Psychopharmacology (Berl). 2007 Aug;193(2):271-82. doi: 10.1007/s00213-007-0784-1. Epub 2007 Apr 15. Psychopharmacology (Berl). 2007. PMID: 17435992
-
Measurement of receptor-activated phosphoinositide turnover in rat brain: nonequivalence of inositol phosphate and CDP-diacylglycerol formation.J Neurochem. 1993 Mar;60(3):1087-92. doi: 10.1111/j.1471-4159.1993.tb03258.x. J Neurochem. 1993. PMID: 8382261
-
[Antidepressant drugs and central monoaminergic receptors].Yakubutsu Seishin Kodo. 1985 Dec;5(4):303-19. Yakubutsu Seishin Kodo. 1985. PMID: 2870595 Review. Japanese.
-
The noradrenergic action in antidepressant treatments: pharmacological and clinical aspects.CNS Neurosci Ther. 2011 Dec;17(6):723-32. doi: 10.1111/j.1755-5949.2010.00217.x. Epub 2010 Dec 14. CNS Neurosci Ther. 2011. PMID: 21155988 Free PMC article. Review.
Cited by
-
The Cooperative Relationship between STAT5 and Reactive Oxygen Species in Leukemia: Mechanism and Therapeutic Potential.Cancers (Basel). 2018 Sep 27;10(10):359. doi: 10.3390/cancers10100359. Cancers (Basel). 2018. PMID: 30262727 Free PMC article. Review.
-
New insights into the intracellular distribution pattern of cationic amphiphilic drugs.Sci Rep. 2017 Mar 10;7:44277. doi: 10.1038/srep44277. Sci Rep. 2017. PMID: 28281674 Free PMC article.
-
Antidepressant stimulation of CDP-diacylglycerol synthesis does not require monoamine reuptake inhibition.BMC Neurosci. 2010 Jan 27;11:10. doi: 10.1186/1471-2202-11-10. BMC Neurosci. 2010. PMID: 20105322 Free PMC article.
-
Phospholipase C mediates (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-, but not lysergic acid diethylamide (LSD)-elicited head bobs in rabbit medial prefrontal cortex.Brain Res. 2013 Jan 23;1491:98-108. doi: 10.1016/j.brainres.2012.10.057. Epub 2012 Nov 1. Brain Res. 2013. PMID: 23123701 Free PMC article.
-
Effects of paroxetine on spatial memory function and protein kinase C expression in a rat model of depression.Exp Ther Med. 2015 Oct;10(4):1489-1492. doi: 10.3892/etm.2015.2663. Epub 2015 Jul 28. Exp Ther Med. 2015. PMID: 26622512 Free PMC article.
References
-
- Waslick BD, Kandel R, Kakouros A. Depression in children and adolescents: an overview. In: Shaffer D and Waslick BD, editor. The many faces of depression in children and adolescents. Washington DC, American Psychiatric Publishing, Inc.; 2002. pp. 1–29.
-
- Ciraulo DA, Tsirulnik-Barts L, Shader RI, Greenblatt DJ. Clinical pharmacology and therapeutics of antidepressants. In: Ciraulo DA and Shader RI, editor. Pharmacotherapy of depression. Totowa NJ, Humana Press; 2004. pp. 33–119.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

