Diaphragm adaptations in patients with COPD
- PMID: 18218129
- PMCID: PMC2248576
- DOI: 10.1186/1465-9921-9-12
Diaphragm adaptations in patients with COPD
Abstract
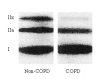
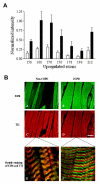
Inspiratory muscle weakness in patients with COPD is of major clinical relevance. For instance, maximum inspiratory pressure generation is an independent determinant of survival in severe COPD. Traditionally, inspiratory muscle weakness has been ascribed to hyperinflation-induced diaphragm shortening. However, more recently, invasive evaluation of diaphragm contractile function, structure, and biochemistry demonstrated that cellular and molecular alterations occur, of which several can be considered pathologic of nature. Whereas the fiber type shift towards oxidative type I fibers in COPD diaphragm is regarded beneficial, rendering the overloaded diaphragm more resistant to fatigue, the reduction of diaphragm fiber force generation in vitro likely contributes to diaphragm weakness. The reduced diaphragm force generation at single fiber level is associated with loss of myosin content in these fibers. Moreover, the diaphragm in COPD is exposed to oxidative stress and sarcomeric injury. This review postulates that the oxidative stress and sarcomeric injury activate proteolytic machinery, leading to contractile protein wasting and, consequently, loss of force generating capacity of diaphragm fibers in patients with COPD. Interestingly, several of these presumed pathologic alterations are already present early in the course of the disease (GOLD I/II), although these patients appear not limited in their daily life activities. Treatment of diaphragm dysfunction in COPD is complex since its etiology is unclear, but recent findings indicate the ubiquitin-proteasome pathway as a prime target to attenuate diaphragm wasting in COPD.
Figures
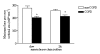

References
-
- Begin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143:905–912. - PubMed
-
- Zielinski J, MacNee W, Wedzicha J, Ambrosino N, Braghiroli A, Dolensky J, Howard P, Gorzelak K, Lahdensuo A, Strom K, Tobiasz M, Weitzenblum E. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis. 1997;52:43–47. - PubMed
-
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:961–966. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

