Polyglutamine expansion reduces the association of TATA-binding protein with DNA and induces DNA binding-independent neurotoxicity
- PMID: 18218637
- PMCID: PMC2276379
- DOI: 10.1074/jbc.M709674200
Polyglutamine expansion reduces the association of TATA-binding protein with DNA and induces DNA binding-independent neurotoxicity
Abstract
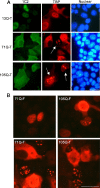
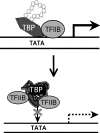
TATA-binding protein (TBP) is essential for eukaryotic gene transcription. Human TBP contains a polymorphic polyglutamine (polyQ) domain in its N terminus and a DNA-binding domain in its highly conserved C terminus. Expansion of the polyQ domain to >42 glutamines typically results in spinocerebellar ataxia type 17 (SCA17), a neurodegenerative disorder that resembles Huntington disease. Our recent studies have demonstrated that polyQ expansion causes abnormal interaction of TBP with the general transcription factor TFIIB and induces neurodegeneration in transgenic SCA17 mice (Friedman, M. J., Shah, A. G., Fang, Z. H., Ward, E. G., Warren, S. T., Li, S., and Li, X. J. (2007) Nat. Neurosci. 10, 1519-1528). However, it remains unknown how polyQ expansion influences DNA binding by TBP. Here we report that polyQ expansion reduces in vitro binding of TBP to DNA and that mutant TBP fragments lacking an intact C-terminal DNA-binding domain are present in transgenic SCA17 mouse brains. polyQ-expanded TBP with a deletion spanning part of the DNA-binding domain does not bind DNA in vitro but forms nuclear aggregates and inhibits TATA-dependent transcription activity in cultured cells. When this TBP double mutant is expressed in transgenic mice, it forms nuclear inclusions in neurons and causes early death. These findings suggest that the polyQ tract affects the binding of TBP to promoter DNA and that polyQ-expanded TBP can induce neuronal toxicity independent of its interaction with DNA.
Figures






Similar articles
-
Synergistic Toxicity of Polyglutamine-Expanded TATA-Binding Protein in Glia and Neuronal Cells: Therapeutic Implications for Spinocerebellar Ataxia 17.J Neurosci. 2017 Sep 20;37(38):9101-9115. doi: 10.1523/JNEUROSCI.0111-17.2017. Epub 2017 Aug 18. J Neurosci. 2017. PMID: 28821675 Free PMC article.
-
Transcriptional dysregulation of TrkA associates with neurodegeneration in spinocerebellar ataxia type 17.Hum Mol Genet. 2009 Nov 1;18(21):4141-52. doi: 10.1093/hmg/ddp363. Epub 2009 Jul 30. Hum Mol Genet. 2009. PMID: 19643914 Free PMC article.
-
Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration.Nat Neurosci. 2007 Dec;10(12):1519-28. doi: 10.1038/nn2011. Epub 2007 Nov 11. Nat Neurosci. 2007. PMID: 17994014
-
Molecular Mechanisms and Therapeutics for SCA17.Neurotherapeutics. 2019 Oct;16(4):1097-1105. doi: 10.1007/s13311-019-00762-z. Neurotherapeutics. 2019. PMID: 31317427 Free PMC article. Review.
-
Molecular Mechanisms of Spinocerebellar Ataxia Type 17.Mol Neurobiol. 2025 May;62(5):5720-5729. doi: 10.1007/s12035-024-04645-z. Epub 2024 Nov 30. Mol Neurobiol. 2025. PMID: 39614971 Review.
Cited by
-
Modulation of Molecular Chaperones in Huntington's Disease and Other Polyglutamine Disorders.Mol Neurobiol. 2017 Oct;54(8):5829-5854. doi: 10.1007/s12035-016-0120-z. Epub 2016 Sep 22. Mol Neurobiol. 2017. PMID: 27660272 Review.
-
Role of the CCAAT-binding protein NFY in SCA17 pathogenesis.PLoS One. 2012;7(4):e35302. doi: 10.1371/journal.pone.0035302. Epub 2012 Apr 17. PLoS One. 2012. PMID: 22530004 Free PMC article.
-
Genetically modified rodent models of SCA17.J Neurosci Res. 2017 Aug;95(8):1540-1547. doi: 10.1002/jnr.23984. Epub 2016 Nov 18. J Neurosci Res. 2017. PMID: 27859490 Free PMC article. Review.
-
A Severe Dementia Syndrome Caused by Intron Retention and Cryptic Splice Site Activation in STUB1 and Exacerbated by TBP Repeat Expansions.Front Mol Neurosci. 2022 Apr 14;15:878236. doi: 10.3389/fnmol.2022.878236. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35493319 Free PMC article.
-
Functional implications of paralog genes in polyglutamine spinocerebellar ataxias.Hum Genet. 2023 Dec;142(12):1651-1676. doi: 10.1007/s00439-023-02607-4. Epub 2023 Oct 16. Hum Genet. 2023. PMID: 37845370 Free PMC article. Review.
References
-
- Hochheimer, A., and Tjian, R. (2003) Genes Dev. 17 1309–1320 - PubMed
-
- Sandelin, A., Carninci, P., Lenhard, B., Ponjavic, J., Hayashizaki, Y., and Hume, D. A. (2007) Nat. Rev. 8 424–436 - PubMed
-
- Lee, T. I., and Young, R. A. (2000) Annu. Rev. Genet. 34 77–137 - PubMed
-
- Baek, H. J., Kang, Y. K., and Roeder, R. G. (2006) J. Biol. Chem. 281 15172–15181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

