Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats
- PMID: 18218678
- PMCID: PMC2375698
- DOI: 10.1113/jphysiol.2007.149625
Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats
Abstract
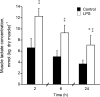
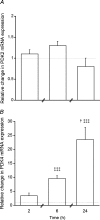
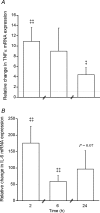
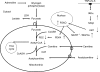
A characteristic manifestation of sepsis is muscle lactate accumulation. This study examined any putative (causative) association between pyruvate dehydrogenase complex (PDC) inhibition and lactate accumulation in the extensor digitorum longus (EDL) muscle of rats infused with lipopolysaccharide (LPS), and explored the involvement of increased transcription of muscle-specific pyruvate dehydrogenase kinase (PDK) isoenzymes. Conscious, male Sprague-Dawley rats were infused i.v. with saline (0.4 ml h(-1), control) or LPS (150 mug kg(-1) h(-1)) for 2 h, 6 h or 24 h (n = 6-8). Muscle lactate concentration was elevated after 2, 6 and 24 h LPS infusion. Muscle PDC activity was the same at 2 h and 6 h, but was 65% lower after 24 h of LPS infusion (P < 0.01), when there was a 47% decrease in acetylcarnitine concentration (P < 0.05), and a 24-fold increase in PDK4 mRNA expression (P < 0.001). These changes were preceded by marked increases in tumour necrosis factor-alpha and interleukin-6 mRNA expression at 2 h. The findings indicate that the early (2 and 6 h) elevation in muscle lactate concentration during LPS infusion was not attributable to limited muscle oxygen availability or ATP production (evidenced by unchanged ATP and phosphocreatine (PCr) concentrations) or to PDC inhibition, whereas after 24 h, muscle lactate accumulation appears to have resulted from PDC activation status limiting pyruvate flux, most probably due to cytokine-mediated up-regulation of PDK4 transcription.
Figures

References
-
- Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–297. - PubMed
-
- Blair R, Fishman B, Amit Z, Weeks JR. A simple double channel swivel for infusions of fluids into unrestrained animals. Pharmacol Biochem Behav. 1980;12:463–466. - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. - PubMed
-
- Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–R497. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

