Protein structure and oligomerization are important for the formation of export-competent HIV-1 Rev-RRE complexes
- PMID: 18218716
- PMCID: PMC2248316
- DOI: 10.1110/ps.073246608
Protein structure and oligomerization are important for the formation of export-competent HIV-1 Rev-RRE complexes
Abstract
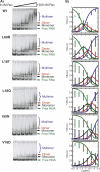
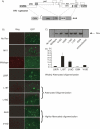
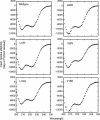
The translation of the unspliced and partially spliced viral mRNAs that encode the late, structural proteins of HIV-1 depends on the viral-protein Rev. Oligomeric binding of Rev to the Rev response element (RRE) in these mRNAs promotes their export from the nucleus and thus controls their expression. Here, we compared the effects of hydrophobic to hydrophilic mutations within the oligomerization domain of Rev using assays for oligomeric RNA binding, protein structure, and export from the nucleus. Oligomeric RNA binding alone does not correlate well with RNA transport activity in the subset of mutants. However, protein structure as judged by CD spectroscopy does correlate well with Rev function. The oligomeric assembly of Rev-L18T is impaired but exhibits minor defects in structure and retains a basal level of activity in vivo. The prevalence of L18T in infected individuals suggests a positive selection mechanism for L18T modulation of Rev activity that may delay the onset of AIDS.
Figures
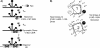


References
-
- Andersen, N.H., Liu, Z., Prickett, K.S. Efforts toward deriving the CD spectrum of a 3(10) helix in aqueous medium. FEBS Lett. 1996;399:47–52. - PubMed
-
- Andrade, M.A., Chacon, P., Merelo, J.J., Moran, F. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 1993;6:383–390. - PubMed
-
- Auer, M., Gremlich, H.U., Seifert, J.M., Daly, T.J., Parslow, T.G., Casari, G., Gstach, H. Helix-loop-helix motif in HIV-1 Rev. Biochemistry. 1994;33:2988–2996. - PubMed
-
- Aukrust, I., Evensen, L., Hollas, H., Berven, F., Atkinson, R.A., Trave, G., Flatmark, T., Vedeler, A. Engineering, biophysical characterisation and binding properties of a soluble mutant form of annexin A2 domain IV that adopts a partially folded conformation. J. Mol. Biol. 2006;363:469–481. - PubMed
-
- Bevec, D., Jaksche, H., Oft, M., Wohl, T., Himmelspach, M., Pacher, A., Schebesta, M., Koettnitz, K., Dobrovnik, M., Csonga, R., et al. Inhibition of HIV-1 replication in lymphocytes by mutants of the Rev cofactor eIF-5A. Science. 1996;271:1858–1860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
