Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects
- PMID: 18218820
- PMCID: PMC3638035
- DOI: 10.1634/stemcells.2007-0843
Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects
Abstract
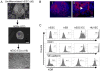
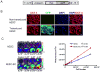
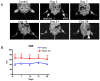
Human embryonic stem (hES) cells are pluripotent stem cells capable of self-renewal and differentiation into virtually all cell types. Thus, they hold tremendous potential as cell sources for regenerative therapies. The concurrent development of accurate, sensitive, and noninvasive technologies capable of monitoring hES cells engraftment in vivo can greatly expedite basic research prior to future clinical translation. In this study, hES cells were stably transduced with a lentiviral vector carrying a novel double-fusion reporter gene that consists of firefly luciferase and enhanced green fluorescence protein. Reporter gene expression had no adverse effects on cell viability, proliferation, or differentiation to endothelial cells (human embryonic stem cell-derived endothelial cells [hESC-ECs]). To compare the two popular imaging modalities, hES cells and hESC-ECs were then colabeled with superparamagnetic iron oxide particles before transplantation into murine hind limbs. Longitudinal magnetic resonance (MR) imaging showed persistent MR signals in both cell populations that lasted up to 4 weeks. By contrast, bioluminescence imaging indicated divergent signal patterns for hES cells and hESC-ECs. In particular, hESC-ECs showed significant bioluminescence signals at day 2, which decreased progressively over the following 4 weeks, whereas bioluminescence signals from undifferentiated hES cells increased dramatically during the same period. Post-mortem histology and immunohistochemistry confirmed teratoma formation after injection of undifferentiated hES cells but not hESC-ECs. From these data taken together, we concluded that reporter gene is a better marker for monitoring cell viability, whereas iron particle labeling is a better marker for high-resolution detection of cell location by MR. Furthermore, transplantation of predifferentiated rather than undifferentiated hES cells would be more suited for avoiding teratoma formation.
Figures


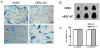


Similar articles
-
Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases.J Cell Biochem. 2009 Feb 1;106(2):194-9. doi: 10.1002/jcb.22003. J Cell Biochem. 2009. PMID: 19097085 Free PMC article. Review.
-
Bioluminescence reporter gene imaging characterize human embryonic stem cell-derived teratoma formation.J Cell Biochem. 2011 Mar;112(3):840-8. doi: 10.1002/jcb.22982. J Cell Biochem. 2011. PMID: 21328457 Free PMC article.
-
In vitro and in vivo bioluminescence reporter gene imaging of human embryonic stem cells.J Vis Exp. 2008 May 2;(14):740. doi: 10.3791/740. J Vis Exp. 2008. PMID: 19066577 Free PMC article.
-
Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction.PLoS One. 2009 Dec 31;4(12):e8443. doi: 10.1371/journal.pone.0008443. PLoS One. 2009. PMID: 20046878 Free PMC article.
-
Technology insight: in vivo cell tracking by use of MRI.Nat Clin Pract Cardiovasc Med. 2006 Oct;3(10):554-62. doi: 10.1038/ncpcardio0659. Nat Clin Pract Cardiovasc Med. 2006. PMID: 16990841 Review.
Cited by
-
Development of new technologies for stem cell research.J Biomed Biotechnol. 2012;2012:741416. doi: 10.1155/2012/741416. Epub 2012 Nov 26. J Biomed Biotechnol. 2012. PMID: 23251081 Free PMC article. Review.
-
A Dual Gold Nanoparticle System for Mesenchymal Stem Cell Tracking.J Mater Chem B. 2014 Dec 14;2(46):8220-8230. doi: 10.1039/C4TB00975D. J Mater Chem B. 2014. PMID: 25709814 Free PMC article.
-
In vivo magnetic resonance imaging of injected endothelial progenitor cells after myocardial infarction in rats.Mol Imaging Biol. 2011 Apr;13(2):303-13. doi: 10.1007/s11307-010-0359-0. Mol Imaging Biol. 2011. PMID: 20552286
-
PTH Induces Systemically Administered Mesenchymal Stem Cells to Migrate to and Regenerate Spine Injuries.Mol Ther. 2016 Feb;24(2):318-330. doi: 10.1038/mt.2015.211. Epub 2015 Nov 20. Mol Ther. 2016. PMID: 26585691 Free PMC article.
-
Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases.J Cell Biochem. 2009 Feb 1;106(2):194-9. doi: 10.1002/jcb.22003. J Cell Biochem. 2009. PMID: 19097085 Free PMC article. Review.
References
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. - PubMed
-
- Li ZJ, et al. Kinetic expression of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) during embryonic stem cell differentiation. J Cell Biochem. 2005;95(3):559–70. - PubMed
-
- Reubinoff BE, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1134–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

