Altered intracellular and extracellular signaling leads to impaired T-cell functions in ADA-SCID patients
- PMID: 18218852
- PMCID: PMC2288726
- DOI: 10.1182/blood-2007-05-092429
Altered intracellular and extracellular signaling leads to impaired T-cell functions in ADA-SCID patients
Erratum in
- Blood. 2014 Jun 5;123(23):3682
Abstract
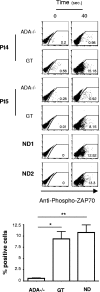
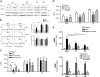
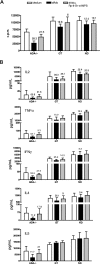
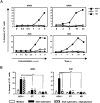
Mutations in the adenosine deaminase (ADA) gene are responsible for a form of severe combined immunodeficiency (SCID) caused by the lymphotoxic accumulation of ADA substrates, adenosine and 2'-deoxy-adenosine. The molecular mechanisms underlying T-cell dysfunction in humans remain to be elucidated. Here, we show that CD4(+) T cells from ADA-SCID patients have severely compromised TCR/CD28-driven proliferation and cytokine production, both at the transcriptional and protein levels. Such an impairment is associated with an intrinsically reduced ZAP-70 phosphorylation, Ca(2+) flux, and ERK1/2 signaling and to defective transcriptional events linked to CREB and NF-kappaB. Moreover, exposure to 2'-deoxy-adenosine results in a stronger inhibition of T-cell activation, mediated by the aberrant A(2A) adenosine receptor signaling engagement and PKA hyperactivation, or in a direct apoptotic effect at higher doses. Conversely, in T cells isolated from patients after gene therapy with retrovirally transduced hematopoietic stem/progenitor cells, the biochemical events after TCR triggering occur properly, leading to restored effector functions and normal sensitivity to apoptosis. Overall, our findings provide a better understanding of the pathogenesis of the immune defects associated with an altered purine metabolism and confirm that ADA gene transfer is an efficacious treatment for ADA-SCID. The trials in this study are enrolled at www.ClinicalTrials.gov as #NCT00598481 and #NCT0059978.
Trial registration: ClinicalTrials.gov NCT00598481 NCT00599781.
Figures


References
-
- Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1069. - PubMed
-
- Buckley RH, Schiff RI, Schiff SE, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–387. - PubMed
-
- Hirschhorn R. Immunodeficiency disease due to deficiency of adenosine deaminase. In: Ochs H, Smith C, Puck J, editors. Primary Immunodeficiency Diseases. New York, NY: Oxford Univ. Press; 1999.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

