Structural and functional effects of hereditary hemolytic anemia-associated point mutations in the alpha spectrin tetramer site
- PMID: 18218854
- PMCID: PMC2424163
- DOI: 10.1182/blood-2007-11-122457
Structural and functional effects of hereditary hemolytic anemia-associated point mutations in the alpha spectrin tetramer site
Abstract
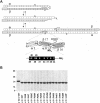
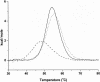
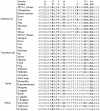
The most common hereditary elliptocytosis (HE) and hereditary pyropoikilocytosis (HPP) mutations are alpha-spectrin missense mutations in the dimer-tetramer self-association site. In this study, we systematically compared structural and functional properties of the 14 known HE/HPP mutations located in the alpha-spectrin tetramer binding site. All mutant alpha-spectrin recombinant peptides were well folded, stable structures, with only the R34W mutant exhibiting a slight structural destabilization. In contrast, binding affinities measured by isothermal titration calorimetry were greatly variable, ranging from no detectable binding observed for I24S, R28C, R28H, R28S, and R45S to approximately wild-type binding for R34W and K48R. Binding affinities for the other 7 mutants were reduced by approximately 10- to 100-fold relative to wild-type binding. Some sites, such as R28, were hot spots that were very sensitive to even relatively conservative substitutions, whereas other sites were only moderately perturbed by nonconservative substitutions. The R34W and K48R mutations were particularly intriguing mutations that apparently either destabilize tetramers through mechanisms not probed by the univalent tetramer binding assay or represent polymorphisms rather than the pathogenic mutations responsible for observed clinical symptoms. All alpha0 HE/HPP mutations studied here appear to exert their destabilizing effects through molecular recognition rather than structural mechanisms.
Figures

Comment in
-
Mechanisms of elliptocytosis: significant spectrin substitutions.Blood. 2008 Jun 15;111(12):5417. doi: 10.1182/blood-2008-02-137828. Blood. 2008. PMID: 18544686 No abstract available.
Similar articles
-
The common hereditary elliptocytosis-associated α-spectrin L260P mutation perturbs erythrocyte membranes by stabilizing spectrin in the closed dimer conformation.Blood. 2013 Oct 24;122(17):3045-53. doi: 10.1182/blood-2013-02-487702. Epub 2013 Aug 23. Blood. 2013. PMID: 23974198 Free PMC article.
-
Molecular basis and haplotyping of the alphaII domain polymorphisms of spectrin: application to the study of hereditary elliptocytosis and pyropoikilocytosis.Am J Hum Genet. 1996 Aug;59(2):351-9. Am J Hum Genet. 1996. PMID: 8755921 Free PMC article.
-
A common type of the spectrin alpha I 46-50a-kD peptide abnormality in hereditary elliptocytosis and pyropoikilocytosis is associated with a mutation distant from the proteolytic cleavage site. Evidence for the functional importance of the triple helical model of spectrin.J Clin Invest. 1992 Mar;89(3):892-8. doi: 10.1172/JCI115669. J Clin Invest. 1992. PMID: 1541680 Free PMC article.
-
Clinical expression of alpha spectrin mutants in hereditary elliptocytosis.Blood Cells. 1987;13(1-2):237-50. Blood Cells. 1987. PMID: 3311220 Review.
-
Hereditary elliptocytosis, spherocytosis and related disorders: consequences of a deficiency or a mutation of membrane skeletal proteins.Blood Rev. 1987 Sep;1(3):147-68. doi: 10.1016/0268-960x(87)90031-2. Blood Rev. 1987. PMID: 3332099 Review.
Cited by
-
Understanding pathogenic single-nucleotide polymorphisms in multidomain proteins--studies of isolated domains are not enough.FEBS J. 2013 Feb;280(4):1018-27. doi: 10.1111/febs.12094. Epub 2013 Jan 16. FEBS J. 2013. PMID: 23241237 Free PMC article.
-
Abnormalities of the erythrocyte membrane.Pediatr Clin North Am. 2013 Dec;60(6):1349-62. doi: 10.1016/j.pcl.2013.09.001. Epub 2013 Oct 15. Pediatr Clin North Am. 2013. PMID: 24237975 Free PMC article. Review.
-
Red cell membrane: past, present, and future.Blood. 2008 Nov 15;112(10):3939-48. doi: 10.1182/blood-2008-07-161166. Blood. 2008. PMID: 18988878 Free PMC article. Review.
-
Conformational changes at the tetramerization site of erythroid alpha-spectrin upon binding beta-spectrin: a spin label EPR study.Biochemistry. 2008 Oct 7;47(40):10765-72. doi: 10.1021/bi800840p. Epub 2008 Sep 11. Biochemistry. 2008. PMID: 18783249 Free PMC article.
-
The common hereditary elliptocytosis-associated α-spectrin L260P mutation perturbs erythrocyte membranes by stabilizing spectrin in the closed dimer conformation.Blood. 2013 Oct 24;122(17):3045-53. doi: 10.1182/blood-2013-02-487702. Epub 2013 Aug 23. Blood. 2013. PMID: 23974198 Free PMC article.
References
-
- Tse WT, Lux SE. Red blood cell membrane disorders. Br J Haematol. 1999;104:2–13. - PubMed
-
- Iolascon A, Perrotta S, Stewart GW. Red blood cell membrane defects. Rev Clin Exp Hematol. 2003;7:22–56. - PubMed
-
- Gallagher PG. Hereditary elliptocytosis: spectrin and protein 4.1R. Semin Hematol. 2004;41:142–164. - PubMed
-
- Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21:1–20. - PubMed
-
- Speicher DW, Marchesi VT. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984;311:177–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

