Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens
- PMID: 18218974
- PMCID: PMC2259047
- DOI: 10.1104/pp.107.110544
Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens
Abstract
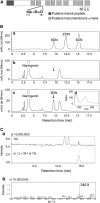
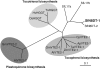
Prenylated flavonoids are natural compounds that often represent the active components in various medicinal plants and exhibit beneficial effects on human health. Prenylated flavonoids are hybrid products composed of a flavonoid core mainly attached to either 5-carbon (dimethylallyl) or 10-carbon (geranyl) prenyl groups derived from isoprenoid (terpenoid) metabolism, and the prenyl groups are crucial for their biological activity. Prenylation reactions in vivo are crucial coupling processes of two major metabolic pathways, the shikimate-acetate and isoprenoid pathways, in which these reactions are also known as a rate-limiting step. However, none of the genes responsible for the prenylation of flavonoids has been identified despite more than 30 years of research in this field. We have isolated a prenyltransferase gene from Sophora flavescens, SfN8DT-1, responsible for the prenylation of the flavonoid naringenin at the 8-position, which is specific for flavanones and dimethylallyl diphosphate as substrates. Phylogenetic analysis shows that SfN8DT-1 has the same evolutionary origin as prenyltransferases for vitamin E and plastoquinone. The gene expression of SfN8DT-1 is strictly limited to the root bark where prenylated flavonoids are solely accumulated in planta. The ectopic expression of SfN8DT-1 in Arabidopsis thaliana resulted in the formation of prenylated apigenin, quercetin, and kaempferol, as well as 8-prenylnaringenin. SfN8DT-1 represents the first flavonoid-specific prenyltransferase identified in plants and paves the way for the identification and characterization of further genes responsible for the production of this large and important class of secondary metabolites.
Figures




References
-
- Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, Bates SE, Castanys S, Gamarro F, Di Pietro A, Perez-Victoria JM (2005) Flavonoid structure-activity studies identify 6-prenyichrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res 65 4852–4860 - PubMed
-
- Barron D, Ibrahim RK (1996) Isoprenylated flavonoids: a survey. Phytochemistry 43 921–982
-
- Biggs DR, Welle R, Grisebach H (1990) Intracellular of prenyltransferases of isoflavonoid phytoalexin biosynthesis in bean and soybean. Planta 181 244–248 - PubMed
-
- Botta B, Vitali A, Menendez P, Misiti D, Delle Monache G (2005) Prenylated flavonoids: pharmacology and biotechnology. Curr Med Chem 12 713–739 - PubMed
-
- Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol 21 1082–1087 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

