Tunnel propagation of postshock activations as a hypothesis for fibrillation induction and isoelectric window
- PMID: 18218982
- PMCID: PMC2868378
- DOI: 10.1161/CIRCRESAHA.107.168112
Tunnel propagation of postshock activations as a hypothesis for fibrillation induction and isoelectric window
Abstract
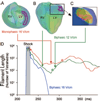
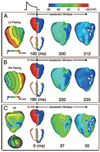
Comprehensive understanding of the ventricular response to shocks is the approach most likely to succeed in reducing defibrillation threshold. We propose a new theory of shock-induced arrhythmogenesis that unifies all known aspects of the response of the heart to monophasic (MS) and biphasic (BS) shocks. The central hypothesis is that submerged "tunnel" propagation of postshock activations through shock-induced intramural excitable areas underlies fibrillation induction and the existence of isoelectric window. We conducted simulations of fibrillation induction using a realistic bidomain model of rabbit ventricles. Following pacing, MS and BS of various strengths/timings were delivered. The results demonstrated that, during the isoelectric window, an activation originated deep within the ventricular wall, arising from virtual electrodes; it then propagated fully intramurally through an excitable tunnel induced by the shock, until it emerged onto the epicardium, becoming the earliest-propagated postshock activation. Differences in shock outcomes for MS and BS were found to stem from the narrower BS intramural postshock excitable area, often resulting in conduction block, and the difference in the mechanisms of origin of the postshock activations, namely intramural virtual electrode-induced phase singularity for MS and virtual electrode-induced propagated graded response for BS. This study provides a novel analysis of the 3D mechanisms underlying the origin of postshock activations in the process of fibrillation induction by MS and BS and the existence of isoelectric window. The tunnel propagation hypothesis could open a new avenue for interventions exploration to achieve significantly lower defibrillation threshold.
Figures





References
-
- Runsio M, Kallner A, Kallner G, Rosenqvist M, Bergfeldt L. Myocardial injury after electrical therapy for cardiac arrhythmias assessed by troponin-T release. Am J Cardiol. 1997;79:1241–1245. - PubMed
-
- Maisel WH. Pacemaker and ICD generator reliability: meta-analysis of device registries. JAMA. 2006;295:1929–1934. - PubMed
-
- Shibata N, Chen PS, Dixon EG, Wolf PD, Danieley ND, Smith WM, Ideker RE. Epicardial activation after unsuccessful defibrillation shocks in dogs. Am J Physiol. 1988;255:H902–H909. - PubMed
-
- Wang NC, Lee MH, Ohara T, Okuyama Y, Fishbein GA, Lin SF, Karagueuzian HS, Chen PS. Optical mapping of ventricular defibrillation in isolated swine right ventricles: demonstration of a postshock isoelectric window after near-threshold defibrillation shocks. Circulation. 2001;104:227–233. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

