NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production
- PMID: 18219313
- PMCID: PMC2267388
- DOI: 10.1038/sj.embor.7401161
NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production
Abstract
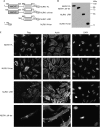
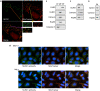
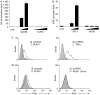
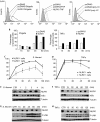
NOD-like receptors (NLRs) are a family of intracellular sensors of microbial- or danger-associated molecular patterns. Here, we report the identification of NLRX1, which is a new member of the NLR family that localizes to the mitochondria. NLRX1 alone failed to trigger most of the common signalling pathways, including nuclear factor-kappaB (NF)-kappaB- and type I interferon-dependent cascades, but could potently trigger the generation of reactive oxygen species (ROS). Importantly, NLRX1 synergistically potentiated ROS production induced by tumour necrosis factor alpha, Shigella infection and double-stranded RNA, resulting in amplified NF-kappaB-dependent and JUN amino-terminal kinases-dependent signalling. Together, these results identify NLRX1 as a NLR that contributes to the link between ROS generation at the mitochondria and innate immune responses.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

