Erythropoietin: when liability becomes asset in neurovascular repair
- PMID: 18219388
- PMCID: PMC2213377
- DOI: 10.1172/JCI34643
Erythropoietin: when liability becomes asset in neurovascular repair
Abstract
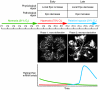
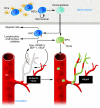
Erythropoietin (Epo) leads to the proliferation and differentiation of erythroid precursors, but is also involved in diverse nonhematopoietic biological functions. In this issue of the JCI, Chen, Smith, and colleagues demonstrate that the temporal expression of Epo is critical for determining whether physiological or pathological repair occurs following neurovascular retinal injury in the oxygen-induced retinopathy neonatal mouse model (see the related article beginning on page 526). The pleiotrophic properties of Epo make it a likely novel therapy for treatment of neurovascular damage, but the timing of its use must be carefully considered to prevent untoward effects.
Figures
Comment on
-
Erythropoietin deficiency decreases vascular stability in mice.J Clin Invest. 2008 Feb;118(2):526-33. doi: 10.1172/JCI33813. J Clin Invest. 2008. PMID: 18219389 Free PMC article.
References
-
- Smith L.E., et al. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. - PubMed
-
- Chen J., Smith L.E. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. - PubMed
-
- Mino R.P., et al. Adenosine receptor antagonists and retinal neovascularization in vivo. Invest. Ophthalmol. Vis. Sci. 2001;42:3320–3324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

