The Tsc/Rheb signaling pathway controls basic amino acid uptake via the Cat1 permease in fission yeast
- PMID: 18219492
- PMCID: PMC2670428
- DOI: 10.1007/s00438-008-0320-y
The Tsc/Rheb signaling pathway controls basic amino acid uptake via the Cat1 permease in fission yeast
Abstract
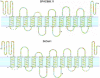
The Tsc/Rheb signaling pathway plays critical roles in the control of growth and cell cycle. Studies in fission yeast have also implicated its importance in the regulation of amino acid uptake. Disruption of tsc2+, one of the tsc+ genes, has been shown to result in decreased arginine uptake and resistance to canavanine. A similar effect is also seen with other basic amino acids. We have identified a permease responsible for the uptake of basic amino acids by genetic complementation and disruption. SPAC869.11 (termed Cat1 for cationic amino acid transporter) contains 12 predicted transmembrane domains and its overexpression in wild type fission yeast leads to the increased uptake of basic amino acids and sensitivity to canavanine. Disruption of cat1+ in the deltatsc2 background interfered with the suppression of the canavanine-resistant phenotype of Atsc2 mutants by a dominant negative Rheb. In deltatsc2 mutant strains, the amount of Cat1 was not altered, but instead was mislocalized. This mislocalization was suppressed by the expression of dominant negative Rheb. In addition, we found that the loss of the E3 ubiquitin ligase, Pub1, also restores proper localization. These results provide a crucial link between Tsc/Rheb signaling and the regulation of the basic amino acid permease in fission yeast.
Figures






Similar articles
-
The fission yeast β-arrestin-like protein Any1 is involved in TSC-Rheb signaling and the regulation of amino acid transporters.J Cell Sci. 2013 Sep 1;126(Pt 17):3972-81. doi: 10.1242/jcs.128355. Epub 2013 Jun 26. J Cell Sci. 2013. PMID: 23813957
-
The Birt-Hogg-Dube and tuberous sclerosis complex homologs have opposing roles in amino acid homeostasis in Schizosaccharomyces pombe.J Biol Chem. 2007 Aug 24;282(34):24583-90. doi: 10.1074/jbc.M700857200. Epub 2007 Jun 7. J Biol Chem. 2007. PMID: 17556368
-
Characterization of canavanine-resistance of cat1 and vhc1 deletions and a dominant any1 mutation in fission yeast.PLoS One. 2022 May 31;17(5):e0269276. doi: 10.1371/journal.pone.0269276. eCollection 2022. PLoS One. 2022. PMID: 35639710 Free PMC article.
-
The TSC/Rheb/TOR signaling pathway in fission yeast and mammalian cells: temperature sensitive and constitutive active mutants of TOR.Cell Cycle. 2007 Jul 15;6(14):1692-5. doi: 10.4161/cc.6.14.4478. Epub 2007 May 21. Cell Cycle. 2007. PMID: 17637564 Review.
-
TOR signaling in fission yeast.Crit Rev Biochem Mol Biol. 2008 Jul-Aug;43(4):277-83. doi: 10.1080/10409230802254911. Crit Rev Biochem Mol Biol. 2008. PMID: 18756382 Review.
Cited by
-
The SAGA histone acetyltransferase complex regulates leucine uptake through the Agp3 permease in fission yeast.J Biol Chem. 2012 Nov 2;287(45):38158-67. doi: 10.1074/jbc.M112.411165. Epub 2012 Sep 18. J Biol Chem. 2012. PMID: 22992726 Free PMC article.
-
Constitutive Tor2 Activity Promotes Retention of the Amino Acid Transporter Agp3 at Trans-Golgi/Endosomes in Fission Yeast.PLoS One. 2015 Oct 8;10(10):e0139045. doi: 10.1371/journal.pone.0139045. eCollection 2015. PLoS One. 2015. PMID: 26447710 Free PMC article.
-
Ragulator and GATOR1 complexes promote fission yeast growth by attenuating TOR complex 1 through Rag GTPases.Elife. 2017 Dec 4;6:e30880. doi: 10.7554/eLife.30880. Elife. 2017. PMID: 29199950 Free PMC article.
-
Psk1, an AGC kinase family member in fission yeast, is directly phosphorylated and controlled by TORC1 and functions as S6 kinase.J Cell Sci. 2012 Dec 1;125(Pt 23):5840-9. doi: 10.1242/jcs.111146. Epub 2012 Sep 12. J Cell Sci. 2012. PMID: 22976295 Free PMC article.
-
Characterization of genome-reduced fission yeast strains.Nucleic Acids Res. 2013 May 1;41(10):5382-99. doi: 10.1093/nar/gkt233. Epub 2013 Apr 5. Nucleic Acids Res. 2013. PMID: 23563150 Free PMC article.
References
-
- Ahmad M, Bussey H. Yeast arginine permease: nucleotide sequence of the CAN1 gene. Curr Genet. 1986;10(8):587–592. - PubMed
-
- Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2003;16(10):1105–1112. - PubMed
-
- Aspuria PJ, Sato T, Tamanoi F. The TSC/Rheb/TOR signaling pathway in Wssion yeast and mammalian cells: temperature sensitive and constitutive active mutants of TOR. Cell Cycle. 2007;6(14):1692–1695. - PubMed
-
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for eYcient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14(10):943–951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

