Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced nonsmall-cell lung cancer
- PMID: 18219661
- PMCID: PMC3355966
- DOI: 10.1002/cncr.23282
Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced nonsmall-cell lung cancer
Abstract
Background: The ISEL (Iressa Survival Evaluation in Lung Cancer) clinical trial evaluated the efficacy of gefitinib versus placebo in pretreated nonsmall-cell lung cancer patients. Two different antibodies, scoring systems, and cutoff points of epidermal growth factor receptor (EGFR) protein expression were compared to predict response and survival of enrolled patients.
Methods: EGFR expression was assessed in tumor samples by immunohistochemistry using the Dako EGFR pharmDx kit (scoring percent of tumor cells with positive staining) and Zymed monoclonal antibody clone 31G7 (scoring staining index derived from proportion of positive cells times staining intensity).
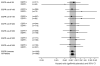
Results: Data for EGFR expression were available for 379 patients for Dako and 357 patients for Zymed antibody (22% and 21%, respectively, of trial population). Objective response rates in gefitinib-treated EGFR-positive patients defined with various cutpoints with Dako antibody varied between 8% and 12%, and with Zymed antibody between 10% and 13%. Lower cutoff points with Dako antibody provided the best discrimination between EGFR-positive and EGFR-negative patients for survival hazard ratios comparing gefitinib to placebo, with a significant treatment/cutoff point interaction for 10% cutoff point (P = .049). A similar but less apparent trend was noted for Zymed antibody, although the discrimination between hazard ratios was not significant for any cutoff point analyzed.
Conclusions: Assessment with the Dako PharmDx kit and percentage of cells with positive staining may provide more accurate prediction of differential effect on survival with gefitinib than assessment with Zymed antibody and staining index. Using higher cutpoints to define positivity does not improve test discrimination.
Figures

Similar articles
-
Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas: Comparison of four commercially available antibodies by immunohistochemistry and fluorescence in situ hybridization study.Lung Cancer. 2010 Jun;68(3):375-82. doi: 10.1016/j.lungcan.2009.07.014. Epub 2009 Aug 26. Lung Cancer. 2010. PMID: 19712993
-
Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS).J Clin Oncol. 2011 Jul 20;29(21):2866-74. doi: 10.1200/JCO.2010.33.4235. Epub 2011 Jun 13. J Clin Oncol. 2011. PMID: 21670455 Clinical Trial.
-
Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer.J Clin Oncol. 2006 Nov 1;24(31):5034-42. doi: 10.1200/JCO.2006.06.3958. J Clin Oncol. 2006. PMID: 17075123 Clinical Trial.
-
The challenge of targeting EGFR: experience with gefitinib in nonsmall cell lung cancer.Eur Respir Rev. 2010 Sep;19(117):186-96. doi: 10.1183/09059180.00005110. Eur Respir Rev. 2010. PMID: 20956191 Free PMC article. Review.
-
Inhibition of the epidermal growth factor receptor in combined modality treatment for locally advanced non-small cell lung cancer.Semin Oncol. 2005 Apr;32(2 Suppl 3):S35-41. doi: 10.1053/j.seminoncol.2005.03.008. Semin Oncol. 2005. PMID: 16015534 Review.
Cited by
-
Requirement for MUC5AC in KRAS-dependent lung carcinogenesis.JCI Insight. 2018 Aug 9;3(15):e120941. doi: 10.1172/jci.insight.120941. eCollection 2018 Aug 9. JCI Insight. 2018. PMID: 30089720 Free PMC article.
-
MicroRNA-27b-3p inhibits the proliferation and invasion of cutaneous squamous cell carcinoma by targeting EGFR and MMP-13.Oncol Lett. 2021 Oct;22(4):729. doi: 10.3892/ol.2021.12990. Epub 2021 Aug 10. Oncol Lett. 2021. PMID: 34429769 Free PMC article.
-
A comprehensive analysis of the expression, epigenetic and genetic changes of HNF1B and ECI2 in 122 cases of high-grade serous ovarian carcinoma.Oncol Lett. 2021 Mar;21(3):185. doi: 10.3892/ol.2021.12446. Epub 2021 Jan 6. Oncol Lett. 2021. PMID: 33574924 Free PMC article.
-
Targeted therapy in non-small-cell lung cancer--is it becoming a reality?Nat Rev Clin Oncol. 2010 Jul;7(7):401-14. doi: 10.1038/nrclinonc.2010.64. Epub 2010 Jun 15. Nat Rev Clin Oncol. 2010. PMID: 20551945 Review.
-
Reproducibility of the EGFR immunohistochemistry scores for tumor samples from patients with advanced non-small cell lung cancer.Oncol Lett. 2017 Feb;13(2):912-920. doi: 10.3892/ol.2016.5512. Epub 2016 Dec 16. Oncol Lett. 2017. PMID: 28356978 Free PMC article.
References
-
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 Trial) J Clin Oncol. 2003;21:2237–2246. - PubMed
-
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. - PubMed
-
- Perez-Soler R. Phase II clinical trial data with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (OSI-774) in non-small-cell lung cancer. Clin Lung Cancer. 2004;6(suppl 1):S20–S23. - PubMed
-
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. - PubMed
-
- Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

