Mammalian collagen IV
- PMID: 18219669
- PMCID: PMC4788096
- DOI: 10.1002/jemt.20564
Mammalian collagen IV
Abstract
Four decades have passed since the first discovery of collagen IV by Kefalides in 1966. Since then collagen IV has been investigated extensively by a large number of research laboratories around the world. Advances in molecular genetics have resulted in identification of six evolutionary related mammalian genes encoding six different polypeptide chains of collagen IV. The genes are differentially expressed during the embryonic development, providing different tissues with specific collagen IV networks each having unique biochemical properties. Newly translated alpha-chains interact and assemble in the endoplasmic reticulum in a chain-specific fashion and form unique heterotrimers. Unlike most collagens, type IV collagen is an exclusive member of the basement membranes and through a complex inter- and intramolecular interactions form supramolecular networks that influence cell adhesion, migration, and differentiation. Collagen IV is directly involved in a number of genetic and acquired disease such as Alport's and Goodpasture's syndromes. Recent discoveries have also highlighted a new and direct role for collagen IV in the development of rare genetic diseases such as cerebral hemorrhage and porencephaly in infants and hemorrhagic stroke in adults. Years of intensive investigations have resulted in a vast body of information about the structure, function, and biology of collagen IV. In this review article, we will summarize essential findings on the structural and functional relationships of different collagen IV chains and their roles in health and disease.
(c) 2008 Wiley-Liss, Inc.
Figures

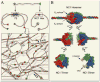
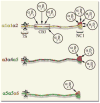
References
-
- Abecassis J, Millon-Collard R, Klein-Soyer C, Nicora F, Fricker JP, Beretz A, Eber M, Muller D, Cazenave JP. Adhesion of human breast cancer cell line MCF-7 to human vascular endothelial cells in culture. Enhancement by activated platelets. Int J Cancer. 1987;40:525–531. - PubMed
-
- Aguglia U, Gambardella A, Breedveld GJ, Oliveri RL, Le Piane E, Messina D, Quattrone A, Heutink P. Suggestive evidence for linkage to chromosome 13qter for autosomal dominant type 1 porencephaly. Neurology. 2004;62:1613–1615. - PubMed
-
- Aumailley M, Gayraud B. Structure and biological activity of the extracellular matrix. J Mol Med. 1998;76:253–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

