Stem cell therapy: MRI guidance and monitoring
- PMID: 18219684
- PMCID: PMC3075622
- DOI: 10.1002/jmri.21263
Stem cell therapy: MRI guidance and monitoring
Abstract
With the recent advances in magnetic resonance (MR) labeling of cellular therapeutics, it is natural that interventional MRI techniques for targeting would be developed. This review provides an overview of the current methods of stem cell labeling and the challenges that are created with respect to interventional MRI administration. In particular, stem cell therapies will require specialized, MR-compatible devices as well as integration of graphical user interfaces with pulse sequences designed for interactive, real-time delivery in many organs. Specific applications that are being developed will be reviewed as well as strategies for future translation to the clinical realm.
(Copyright) 2008 Wiley-Liss, Inc.
Figures

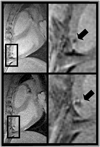
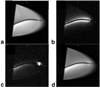
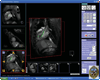




Similar articles
-
Pulse sequences and system interfaces for interventional and real-time MRI.J Magn Reson Imaging. 2008 Feb;27(2):267-75. doi: 10.1002/jmri.21268. J Magn Reson Imaging. 2008. PMID: 18219681 Review.
-
[Interventional MR imaging: state of the art and technological advances].J Radiol. 2008 Jan;89(1 Pt 1):13-20. doi: 10.1016/s0221-0363(08)70365-2. J Radiol. 2008. PMID: 18288022 Review. French.
-
MR-guided endovascular interventions: a comprehensive review on techniques and applications.Eur Radiol. 2008 Apr;18(4):645-57. doi: 10.1007/s00330-007-0818-4. Epub 2007 Dec 11. Eur Radiol. 2008. PMID: 18071710 Review.
-
Intracoronary injection of contrast media maps the territory of the coronary artery: an MRI technique for assessing the effects of locally delivered angiogenic therapies.Acad Radiol. 2008 Nov;15(11):1354-9. doi: 10.1016/j.acra.2008.09.002. Acad Radiol. 2008. PMID: 18995187
-
Targeted drug delivery under MRI guidance.J Magn Reson Imaging. 2008 Feb;27(2):292-8. doi: 10.1002/jmri.21266. J Magn Reson Imaging. 2008. PMID: 18219683 Review.
Cited by
-
Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation.Dis Model Mech. 2009 Jan-Feb;2(1-2):23-38. doi: 10.1242/dmm.001198. Dis Model Mech. 2009. PMID: 19132123 Free PMC article. Review.
-
In vivo imaging of stem cells and Beta cells using direct cell labeling and reporter gene methods.Arterioscler Thromb Vasc Biol. 2009 Jul;29(7):1025-30. doi: 10.1161/ATVBAHA.108.165571. Epub 2009 Apr 9. Arterioscler Thromb Vasc Biol. 2009. PMID: 19359666 Free PMC article. Review.
-
Nanoparticles for cell labeling.Nanoscale. 2011 Jan;3(1):142-53. doi: 10.1039/c0nr00493f. Epub 2010 Oct 11. Nanoscale. 2011. PMID: 20938522 Free PMC article. Review.
-
Human studies of the efficacy and safety of stem cells in the treatment of diabetic peripheral neuropathy: a systematic review and meta-analysis.Stem Cell Res Ther. 2024 Nov 19;15(1):442. doi: 10.1186/s13287-024-04033-3. Stem Cell Res Ther. 2024. PMID: 39563393 Free PMC article.
-
In vivo MRI cell tracking: clinical studies.AJR Am J Roentgenol. 2009 Aug;193(2):314-25. doi: 10.2214/AJR.09.3107. AJR Am J Roentgenol. 2009. PMID: 19620426 Free PMC article. Review.
References
-
- Lewin M, Carlesso N, Tung CH, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. - PubMed
-
- Frank JA, Zywicke H, Jordan EK, et al. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) super-paramagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol. 2002;9 Suppl 2:S484–S487. - PubMed
-
- Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–872. - PubMed
-
- Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. - PubMed
-
- Frank JA, Anderson SA, Kalsih H, et al. Methods for magnetically labeling stem and other cells for detection by in vivo magnetic resonance imaging. Cytotherapy. 2004;6:621–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

