Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study
- PMID: 18220081
- PMCID: PMC2225552
- DOI: 10.1093/sleep/31.1.79
Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study
Abstract
Study objectives: To evaluate long-term efficacy and safety of zolpidem extended-release 3 to 7 nights/week for chronic primary insomnia.
Design: Multicenter, 25-week, phase IIIb, randomized, double-blind, placebo-controlled, parallel-group.
Setting: Outpatient; visits every 4 weeks.
Patients: Aged 18 to 64 years; DSM-IV criteria for chronic primary insomnia; > or =3 months of difficulty initiating or maintaining sleep or experiencing nonrestorative sleep.
Interventions: Single-dose zolpidem extended-release 12.5 mg (n = 669) or placebo (n = 349), self-administered from a minimum of 3 nights/week to a maximum of 7 nights/week.
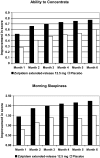
Measurements and results: Patient's Global Impression (PGI) and Clinical Global Impression-Improvement (CGI-I) were assessed every 4 weeks up to week 24. Patient Morning Questionnaire (PMQ), recorded daily, assessed subjective sleep measures-sleep onset latency (SOL), total sleep time (TST), number of awakenings (NAW), wake time after sleep onset (WASO), and quality of sleep (QOS)-and next-day functioning. At week 12, PGI, Item 1 (aid to sleep), the primary endpoint, was scored as favorable (i.e., "helped me sleep") by 89.8% of zolpidem patients vs. 51.4% of placebo patients (P < 0.0001, based on rank score) and at week 24 by 92.3% of zolpidem extended-release patients vs. 59.7% of placebo patients. Zolpidem extended-release also was statistically significantly superior to placebo at every time point for PGI (Items 1-4) and CGI-I (P < 0.0001, rank score), TST, WASO, QOS (P < 0.0001), and SOL (P < or = 0.0014); NAW (Months 2-6; P < 0.0001). Sustained improvement (P < 0.0001, all time points) was observed in morning sleepiness and ability to concentrate (P = 0.0014, month 6) with zolpidem extended-release compared with placebo. Most frequent adverse events for zolpidem extended-release were headache, anxiety and somnolence. No rebound effect was observed during the first 3 nights of discontinuation.
Conclusions: These findings establish the efficacy of 3 to 7 nights per week dosing of zolpidem extended-release 12.5 mg for up to 6 months. Treatment provided sustained and significant improvements in sleep onset and maintenance and also improved next-day concentration and morning sleepiness.
Figures





Comment in
-
Zolpidem extended-release in the long-term treatment of insomnia.Nat Clin Pract Neurol. 2008 Jul;4(7):358-9. doi: 10.1038/ncpneuro0821. Epub 2008 May 20. Nat Clin Pract Neurol. 2008. PMID: 18493240 No abstract available.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Arlington, VA: American Psychiatric Publishing, Inc.; 1994. (DSM-IV)
-
- Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18:163–76. - PubMed
-
- National Institutes of Health. NIH Statement Regarding the Treatment of Insomnia. State of the Science Conference Statement: Manifestations and management of chronic insomnia in adults; June 13-15, 2005. Sleep. 2005;28:1049–57. - PubMed
-
- Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24:425–30. - PubMed
-
- Balter MB, Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. J Clin Psychiatry. 1992;53(Suppl):34–9. - PubMed

