Biological modulation by lectins and their ligands in tumor progression and metastasis
- PMID: 18220503
- PMCID: PMC3794466
- DOI: 10.2174/187152008783330833
Biological modulation by lectins and their ligands in tumor progression and metastasis
Abstract
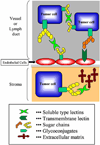
Lectins are a group of specific proteins that preferentially bind to carbohydrates inside and outside cells. To date, an increasing number of animal lectins have been found and categorized into several families in terms of the significant primary structural homology, while the classification is not always straightforward. These lectins can exert immense biological functions mainly through their specific carbohydrate-protein interactions in a variety of situations. In cancer biology, aberrant glycosylation changes on many glycoproteins and glycolipids are often observed and numerous experimental evidences have revealed that these structural changes are related to tumor malignancy. Galectins, which are broadly expressed animal lectins, can play crucial biological roles in tumor cell-cell or cell-matrix interactions through their binding activities to the tumor cell surface carbohydrate determinants. Certain galectin family proteins have also shown to affect tumor cell survival, signal transduction, and proliferation mainly inside the cell. Selectins, which are one of the C-type lectins and expressed leukocytes and/or vascular endothelium, can also play an immense role in tumor cell adhesion and invasion. In addition, certain annexin family proteins, which are originally known as phospholipid binding proteins, have been revealed to possess the carbohydrate binding activity, and these novel functions in tumors are being unveiled. Understanding how carbohydrate-protein interactions function in tumor cells will be one of the important goals in cancer research. This review focuses on the role of these lectins and their ligands in cancer progression and metastasis.
Figures



References
-
- Dennis JW. Semin. Cancer Biol. 1991;2:411. - PubMed
-
- Hakomori S. Cancer Res. 1985;45:2405. - PubMed
-
- Haltiwanger RS, Lowe JB. Annu. Rev. Biochem. 2004;73:491. - PubMed
-
- Eggens I, Fenderson BA, Toyokuni T, Hakomori S. Biochem. Biophys. Res. Commun. 1989;158:913. - PubMed
-
- Hakomori S. Cancer Res. 1996;56:5309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

