Discordant localization of WFA reactivity and brevican/ADAMTS-derived fragment in rodent brain
- PMID: 18221525
- PMCID: PMC2263047
- DOI: 10.1186/1471-2202-9-14
Discordant localization of WFA reactivity and brevican/ADAMTS-derived fragment in rodent brain
Abstract
Background: Proteoglycan (PG) in the extracellular matrix (ECM) of the central nervous system (CNS) may act as a barrier for neurite elongation in a growth tract, and regulate other characteristics collectively defined as structural neural plasticity. Proteolytic cleavage of PGs appears to alter the environment to one favoring plasticity and growth. Brevican belongs to the lectican family of aggregating, chondroitin sulfate (CS)-bearing PGs, and it modulates neurite outgrowth and synaptogenesis. Several ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) are glutamyl-endopeptidases that proteolytically cleave brevican. The purpose of this study was to localize regions of adult CNS that contain a proteolytic-derived fragment of brevican which bears the ADAMTS-cleaved neoepitope sequence. These regions were compared to areas of Wisteria floribunda agglutin (WFA) reactivity, a common reagent used to detect "perineuronal nets" (PNNs) of intact matrix and a marker which is thought to label regions of relative neural stability.
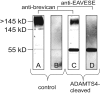
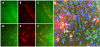
Results: WFA reactivity was found primarily as PNNs, whereas brevican and the ADAMTS-cleaved fragment of brevican were more broadly distributed in neuropil, and in particular regions localized to PNNs. One example is hippocampus where the ADAMTS-cleaved brevican fragment is found surrounding pyramidal neurons, in neuropil of stratum oriens/radiatum and the lacunosum moleculare. The fragment was less abundant in the molecular layer of the dentate gyrus. Mostly PNNs of scattered interneurons along the pyramidal layer were identified by WFA. In lateral thalamus, the reticular thalamic nucleus stained abundantly with WFA whereas ventral posterior nuclei were markedly immunopositive for ADAMTS-cleaved brevican. Using Western blotting techniques, no common species were reactive for brevican and WFA.
Conclusion: In general, a marked discordance was observed in the regional localization between WFA and brevican or the ADAMTS-derived N-terminal fragment of brevican. Functionally, this difference may correspond to regions with varied prevalence for neural stability/plasticity.
Figures






References
-
- Young NM, Williams RE. Assignment of lectins specific for D-galactose or N-acetyl-D-galactosamine to two groups, based on their circular dichroism. Can J Biochem Cell Biol. 1985;63:268–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

